Abstract
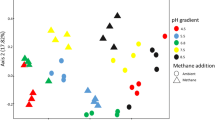
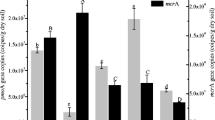
The soil of the former Lake Texcoco is a saline alkaline environment where anthropogenic drainage in some areas has reduced salt content and pH. Potential methane (CH4) consumption rates were measured in three soils of the former Lake Texcoco with different electrolytic conductivity (EC) and pH, i.e. Tex-S1 a >18 years drained soil (EC 0.7 dS m−1, pH 8.5), Tex-S2 drained for ~10 years (EC 9.0 dS m−1, pH 10.3) and the undrained Tex-S3 (EC 84.8 dS m−1, pH 10.3). An arable soil from Alcholoya (EC 0.7 dS m−1, pH 6.7), located nearby Lake Texcoco was used as control. Methane oxidation in the soil Tex-S1 (lowest EC and pH) was similar to that in the arable soil from Alcholoya (32.5 and 34.7 mg CH4 kg−1 dry soil day−1, respectively). Meanwhile, in soils Tex-S2 and Tex-S3, the potential CH4 oxidation rates were only 15.0 and 12.8 mg CH4 kg−1 dry soil day−1, respectively. Differences in CH4 oxidation were also related to changes in the methane-oxidizing communities in these soils. Sequence analysis of pmoA gene showed that soils differed in the identity and number of methanotrophic phylotypes. The Alcholoya soil and Tex-S1 contained phylotypes grouped within the upland soil cluster gamma and the Jasper Ridge, California JR-2 clade. In soil Tex-S3, a phylotype related to Methylomicrobium alcaliphilum was detected.


Similar content being viewed by others
References
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acid Res 25:3389–3402
Antony CP, Kumaresan D, Ferrando L, Boden R, Moussard H, Scavino AF, Shouche YS, Murrell JC (2010) Active methylotrophs in the sediments of Lonar Lake, a saline and alkaline ecosystem formed by meteor impact. ISME J 4:1470–1480
Antony CP, Kumaresan D, Hunger S, Drake HL, Murrell JC, Shouche YS (2012) Microbiology of Lonar Lake and other soda lakes. ISME J 7:468–476
Avnimelech Y, Ritvo G, Meijer LE, Kochba M (2001) Water content, organic carbon and dry bulk density in flooded sediments. Aquac Eng 25:25–33
Bárcena TG, Finster KW, Yde JC (2011) Spatial patterns of soil development, methane oxidation, and methanotrophic diversity along a receding glacier forefield, Southeast Greenland. Arct Antarct Alp Res 43:178–188
Bedard C, Knowles R (1989) Physiology, biochemistry, and specific inhibitors of CH4, NH4 +, and CO oxidation by methanotrophs and nitrifiers. Microbiol Rev 53:68–84
Betancur-Galvis LA, Carrillo H, Luna-Guido M, Marsch R, Dendooven L (2012) Enhanced dissipation of polycyclic aromatic hydrocarbons in the rhizosphere of the Athel Tamarisk (Tamarix aphylla L. Karst.) grown in saline-alkaline soils of the former lake Texcoco. Int J Phytoremediation 14:741–753
Bissett A, Abell GCJ, Bodrossy L, Richardson AE, Thrall PH (2012) Methanotrophic communities in Australian woodland soils of varying salinity. FEMS Microbiol Ecol 80:685–695
Bodrossy L, Holmes EM, Holmes AJ, Kovács KL, Murrell JC (1997) Analysis of 16S rRNA and methane monooxygenase gene sequences reveals a novel group of thermotolerant and thermophilic methanotrophs, Methylocaldum gen. nov. Arch Microbiol 168:493–503
Bourne DG, McDonald IR, Murrell JC (2001) Comparison of pmoA PCR primer sets as tools for investigating methanotroph diversity in three Danish soils. Appl Environ Microbiol 67:3802–3809
Bowman J (2006) The methanotrophs-the families Methylococcaceae and Methylocystaceae Ch. 3.1.14. Prokaryotes 5:266–289
Campanella JJ, Bitincka L, Smalley J (2003) MatGAT: an application that generates similarity/identity matrices using protein or DNA sequences. BMC Bioinform 4:29
Ceja-Navarro JA, Rivera FN, Patiño-Zúñiga L, Govaerts B, Marsch R, Vila-Sanjurjo A, Dendooven L (2010) Molecular characterization of soil bacterial communities in contrasting zero tillage systems. Plant Soil 329:127–137
Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD (2003) Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res 31:3497–3500
Conde E, Cardenas M, Ponce-Mendoza A, Luna-Guido ML, Cruz-Mondragón C, Dendooven L (2005) The impacts of inorganic nitrogen application on mineralization of 14C-labelled maize and glucose, and on priming effect in saline alkaline soil. Soil Biol Biochem 37:681–691
Conrad R, Frenzel P, Cohen Y (1995) Methane emission from hypersaline microbial mats: lack of aerobic methane oxidation activity. FEMS Microbiol Ecol 16:297–305
Costello AM, Lidstrom ME (1999) Molecular characterization of functional and phylogenetic genes from natural populations of methanotrophs in lake sediments. Appl Environ Microbiol 65:5066–5074
Cullen DW, Hirsch PR (1998) Simple and rapid method for direct extraction of microbial DNA from soil for PCR. Soil Biol Biochem 30:983–993
Dalal R, Allen D, Livesley S, Richards G (2008) Magnitude and biophysical regulators of methane emission and consumption in the Australian agricultural, forest, and submerged landscapes: a review. Plant Soil 309:43–76
Dedysh SN, Knief C, Dunfield PF (2005) Methylocella species are facultatively methanotrophic. J Bacteriol 187:4665–4670
Degelmann DM, Borken W, Drake HL, Kolb S (2010) Different atmospheric methane-oxidizing communities in European beech and Norway spruce soils. Appl Environ Microbiol 76:3228–3235
Dendooven L, Alcántara-Hernández RJ, Valenzuela-Encinas C, Luna-Guido ML, Perez-Guevara F, Marsch R (2010) Dynamics of carbon and nitrogen in an extreme alkaline saline soil: a review. Soil Biol Biochem 42:865–877
Dörr N, Glaser B, Kolb S (2010) Methanotrophic communities in Brazilian ferralsols from naturally forested, afforested, and agricultural sites. Appl Environ Microbiol 76:1307–1310
Dumont MG, Murrell JC (2005) Community-level analysis: key genes of aerobic methane oxidation. Methods Enzymol 397:413–427
Eshinimaev BT, Khmelenina VN, Trotsenko YA (2008) First isolation of a type II methanotroph from a soda lake. Microbiology 77:628–631
Fernández-Buces N, Siebe C, Cram S, Palacio JL (2006) Mapping soil salinity using a combined spectral response index for bare soil and vegetation: a case study in the former lake Texcoco, Mexico. J Arid Environ 65:644–667
Galtier N, Gouy M, Gautier C (1996) SEAVIEW and PHYLO_WIN: two graphic tools for sequence alignment and molecular phylogeny. Comput Appl Biosci 12:543–548
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704
Guo C, Sun W, Harsh JB, Ogram A (1997) Hybridization analysis of microbial DNA from fuel oil-contaminated and non contaminated soil. Microb Ecol 34:178–187
Gutiérrez-Castorena MDC, Stoops G, Ortiz-Solorio CA, López-Avila G (2005) Amorphous silica materials in soils and sediments of the Ex-Lago de Texcoco, Mexico: an explanation for its subsidence. Catena 60:205–226
Hakemian AS, Rosenzweig AC (2007) The biochemistry of methane oxidation. Annu Rev Biochem 76:223–241
Han B, Chen Y, Abell G, Jiang H, Bodrossy L, Zhao J, Murrell JC, Xing XH (2009) Diversity and activity of methanotrophs in alkaline soil from a Chinese coal mine. FEMS Microbiol Ecol 70:196–207
Hanson RS, Hanson TE (1996) Methanotrophic bacteria. Microbiol Rev 60:439–471
Holmes AJ, Costello A, Lidstrom ME, Murrell JC (1995) Evidence that particulate methane monooxygenase and ammonia monooxygenase may be evolutionarily related. FEMS Microbiol Lett 132:203–208
Holmes AJ, Roslev P, McDonald IR, Iversen N, Henriksen K, Murrell JC (1999) Characterization of methanotrophic bacterial populations in soils showing atmospheric methane uptake. Appl Environ Microbiol 65:3312–3318
Horz HP, Rich V, Avrahami S, Bohannan BJM (2005) Methane-oxidizing bacteria in a California upland grassland soil: diversity and response to simulated global change. Appl Environ Microbiol 71:2642–2652
Jensen S, Neufeld JD, Birkeland NK, Hovland M, Murrell JC (2008) Methane assimilation and trophic interactions with marine Methylomicrobium in deep-water coral reef sediment off the coast of Norway. FEMS Microbiol Ecol 66:320–330
Jones RD, Morita RY (1983) Methane oxidation by Nitrosococcus oceanus and Nitrosomonas europaea. Appl Environ Microbiol 45:401–410
Jones BE, Grant WD, Duckworth AW, Owenson GG (1998) Microbial diversity of soda lakes. Extremophiles 2:191–200
Khmelenina VN, Kalyuzhnaya MG, Starostina NG, Suzina NE, Trotsenko YA (1997) Isolation and characterization of halotolerant alkaliphilic methanotrophic bacteria from Tuva soda Lakes. Curr Microbiol 35:257–261
Khmelenina VN, Eshinimaev BT, Kalyuzhnaya MG, Trotsenko I (2000) Potential activity of methane and ammonia oxidation by methanotrophic communities from soda lakes of the southern Transbaikal. Mikrobiologiia 69:553–558
Knief C, Lipski A, Dunfield PF (2003) Diversity and activity of methanotrophic bacteria in different upland soils. Appl Environ Microbiol 69:6703–6714
Levine UY, Teal TK, Robertson GP, Schmidt TM (2011) Agriculture’s impact on microbial diversity and associated fluxes of carbon dioxide and methane. ISME J 5:1683–1691
Lin JL, Radajewski S, Eshinimaev BT, Trotsenko YA, McDonald IR, Murrell JC (2004) Molecular diversity of methanotrophs in Transbaikal soda lake sediments and identification of potentially active populations by stable isotope probing. Environ Microbiol 6:1049–1060
Lin JL, Joye SB, Scholten JC, Schafer H, McDonald IR, Murrell JC (2005) Analysis of methane monooxygenase genes in mono lake suggests that increased methane oxidation activity may correlate with a change in methanotroph community structure. Appl Environ Microbiol 71:6458–6462
Lüke C, Frenzel P (2011) Potential of pmoA amplicon pyrosequencing for methanotroph diversity studies. Appl Environ Microbiol 77:6305–6309
Lüke C, Krause S, Cavigiolo S, Greppi D, Lupotto E, Frenzel P (2010) Biogeography of wetland rice methanotrophs. Environ Microbiol 12:862–872
Luna-Guido ML, Beltrán-Hernández RI, Solís-Ceballos NA, Hernández-Chávez N, Mercado-García F, Catt JA, Olalde-Portugal V, Dendooven L (2000) Chemical and biological characteristics of alkaline saline soils from the former Lake Texcoco as affected by artificial drainage. Biol Fertil Soils 32:102–108
McDonald IR, Bodrossy L, Chen Y, Murrell JC (2008) Molecular ecology techniques for the study of aerobic methanotrophs. Appl Environ Microbiol 74:1305–1315
Murrell JC, Gilbert B, McDonald IR (2000) Molecular biology and regulation of methane monooxygenase. Arch Microbiol 173:325–332
Nakamura T, Hoaki T, Hanada S, Maruyama A, Kamagata Y, Fuse H (2007) Soluble and particulate methane monooxygenase gene clusters in the marine methanotroph Methylomicrobium sp. strain NI. FEMS Microbiol Lett 277:157–164
Op den Camp H, Islam T, Stott M, Harhangi H, Hynes A, Schouten S, Jetten M, Birkeland N-K, Pol A, Dunfield P (2009) Environmental, genomic and taxonomic perspectives on methanotrophic Verrucomicrobia. Environ Microbiol Rep 1:293–306
Pester M, Friedrich MW, Schink B, Brune A (2004) pmoA-based analysis of methanotrophs in a littoral lake sediment reveals a diverse and stable community in a dynamic environment. Appl Environ Microbiol 70:3138–3142
Rambaut A (2007) FigTree, a graphical viewer of phylogenetic trees. http://tree.bio.ed.ac.uk/software/figtree/
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541
Semrau JD, DiSpirito AA, Yoon S (2010) Methanotrophs and copper. FEMS Microbiol Rev 34:496–531
Serrano-Silva N, Luna-Guido M, Fernández-Luqueño F, Marsch R, Dendooven L (2011) Emission of greenhouse gases from an agricultural soil amended with urea: a laboratory study. Appl Soil Ecol 47:92–97
Singh BK, Tate KR, Ross DJ, Singh J, Dando J, Thomas N, Millard P, Murrell JC (2009) Soil methane oxidation and methanotroph responses to afforestation of pastures with Pinus radiata stands. Soil Biol Biochem 41:2196–2205
Smith KA, Ball T, Conen F, Dobbie KE, Massheder J, Rey A (2003) Exchange of greenhouse gases between soil and atmosphere: interactions of soil physical factors and biological processes. Eur J Soil Sci 54:779–791
Sorokin DY, Kuenen JG (2005) Chemolithotrophic haloalkaliphiles from soda lakes. FEMS Microbiol Ecol 52:287–295
Sorokin DY, Jones BE, Kuenen JG (2000) A novel obligately methylotrophic, methane-oxidizing Methylomicrobium species from a highly alkaline environment. Extremophiles 4:145–155
Stoecker K, Bendinger B, Schöning B, Nielsen PH, Nielsen JL, Baranyi C, Toenshoff ER, Daims H, Wagner M (2006) Cohn’s Crenothrix is a filamentous methane oxidizer with an unusual methane monooxygenase. Proc Natl Acad Sci USA 103:2363–2367
Trotsenko YA, Khmelenina VN (2002) Biology of extremophilic and extremotolerant methanotrophs. Arch Microbiol 177:123–131
Trotsenko YA, Murrell JC (2008) Metabolic aspects of aerobic obligate methanotrophy. Adv Appl Microbiol 63:183–229
Trotsenko YA, Medvedkova KA, Khmelenina VN, Eshinimayev BT (2009) Thermophilic and thermotolerant aerobic methanotrophs. Microbiology 78:387–401
Valenzuela-Encinas C, Neria-González I, Alcántara-Hernández R, Enríquez-Aragón J, Estrada-Alvarado I, Hernández-Rodríguez C, Dendooven L, Marsch R (2008) Phylogenetic analysis of the archaeal community in an alkaline–saline soil of the former lake Texcoco (Mexico). Extremophiles 12:247–254
Valenzuela-Encinas C, Neria-Gonzalez I, Alcantara-Hernandez RJ, Estrada-Alvarado I, de la Serna FJZD, Dendooven L, Marsch R (2009) Changes in the bacterial populations of the highly alkaline saline soil of the former lake Texcoco (Mexico) following flooding. Extremophiles 13:609–621
Valenzuela-Encinas C, Alcántara-Hernández RJ, Estrada-Alvarado I, de la Serna FJZD, Dendooven L, Marsch R (2012) The archaeal diversity and population in a drained alkaline saline soil of the former lake Texcoco (Mexico). Geomicrobiology 29:18–22
Völker H, Schweisfurth R, Hirsch P (1977) Morphology and ultrastructure of Crenothrix polyspora Cohn. J Bacteriol 131:306–313
Vorobev AV, Baani M, Doronina NV, Brady AL, Liesack W, Dunfield PF, Dedysh SN (2011) Methyloferula stellata gen. nov., sp. nov., an acidophilic, obligately methanotrophic bacterium that possesses only a soluble methane monooxygenase. Int J Syst Evol Microbiol 61:2456–2463
Zhang J, Li Z, Ning T, Gu S (2011) Methane uptake in salt-affected soils shows low sensitivity to salt addition. Soil Biol Biochem 43:1434–1439
Acknowledgments
The research was funded by “Consejo Nacional de Ciencia y Tecnología” (CONACYT) project CB‐2010‐01-153216. N. S.-S., R. J. A.-H. and C. V.-E. received grant-aided support from CONACYT.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Oren.
Rights and permissions
About this article
Cite this article
Serrano-Silva, N., Valenzuela-Encinas, C., Marsch, R. et al. Changes in methane oxidation activity and methanotrophic community composition in saline alkaline soils. Extremophiles 18, 561–571 (2014). https://doi.org/10.1007/s00792-014-0641-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-014-0641-1