Abstract
Study was undertaken to determine the effect of hydrostatic pressure on the sorption of pentachlorophenol (PCP). The experiment was conducted at atmospheric pressure (1000 hPa) and at increased pressure (6000 hPa) simulating conditions at the water depth of 50 m. The sorption of PCP was examined in an artificial environment (microcosm) consisting of the marine water and the bottom sediments from a Polish harbor and the southern Baltic Sea. The first part of the experiment comprised the determination of PCP sorption parameters in the microcosms and parameters of the sediments (organic matter content, conductivity) and of the overlying water (pH, ion concentration) at 1000 hPa. The second part of the experiment was conducted at 6000 hPa inside the hyperbaric chamber. The hyperbaric exposure affected parameters of the harbor sediments and the overlying water but had little influence on the concentration of PCP in the microcosms containing the southern Baltic Sea sediments. Considering the specific characteristics of the harbor sediments, it can be assumed that the impact of hydrostatic pressure on the sorption process of PCP at 50-m depth appears to be negligible.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sorption and desorption processes, occurring in the seabed, are important in the marine environmental studies because they affect the composition of marine water and also transport and transformation of chemicals (Hedman et al. 2008; Soubaneh et al. 2015). The effect of hydrostatic pressure on the life processes of bacteria population was examined by numerous researchers (Somero 1992; Schedler et al. 2014; Scoma et al. 2016) but studies on the effect of hydrostatic pressure on sorption of contaminants in sediments have not been undertaken. Typically, sorption experiments are carried out at atmospheric pressure. Therefore, the possible influence of high pressure caused by a water column, i.e., hydrostatic pressure, is not taken into consideration. This force, depending on the water depth, can attain high values and possibly influence the sorption/desorption processes. One of the objectives of this study was to determine whether, and eventually by how much, a pressure increase of 5000 hPa can affect these processes.
Persistent organic pollutants (POPs), including pentachlorophenol (PCP) and its salts and esters, belong to the hydrophobic compounds (The Stockholm Convention 2015). This substance was added to the Stockholm Convention on the basis of adverse effects on humans and animals health, molar mass more than 236 g mol−1 (increased toxicity), the water/octanol partition parameter within 5.0 (a significant bioaccumulation), and large-scale production (U. S. Department of Health and Human Services 2014). Strong sorption process can effectively affect the removal of a substance from the water and immobilize it on/in grains forming the bottom. However, as a result of bioturbation, influence of current fields and changes in pH or temperature desorption followed by secondary pollution can occur (Chorover and Brusseau 2008; Hedman et al. 2009; Maciak et al. 2016).
Sorption is the phenomenon of substance (sorptive) accumulation at the surface (adsorption) of a solid (sorbent) and the phenomenon of uptake of the sorptive by entire sorbent (absorption). Sorption experiments are carried out using two-phase systems: solid-liquid, gas-liquid, or solid-gas. These experimental systems, designed to reflect in a smaller-scale processes occurring in the environment, are called microcosms (Hedman et al. 2008; Olsen et al. 2017). When microcosm consists of solid (I)–liquid (II) system, the matrix can be composed of (I) natural bottom sediments or only its selected fraction (sand, silt), soil, mineral (montmorillonite), and various types of biomasses; and (II) marine or freshwater, deionized water, or artificially created water on the basis of typical electrolytes (CaCl2, MgCl2) with the addition of a buffer solution (Chen et al. 2004; Subramanian et al. 2010; León-Santiesteban et al. 2014; Liping et al. 2015).
To describe the environmental activity and the parameters of given compounds, computer modeling can be applied, for example, quantitative structure-activity relationship (QSAR) method (Puzyn et al. 2008). The source of contaminants can also be detected by statistical methods (Witt 2015).
This study aimed to determine the differences in the kinetics of geochemical processes at room temperature under atmospheric pressure (about 1000 hPa) and pressure increased to 6000 hPa. The conditions in microcosms at the raised pressure corresponded to the conditions at a water depth of 50 m. The research hypothesis states that force of increased hydrostatic pressure at a depth of 50 m should accelerate sorption process of pentachlorophenol on sediments, compared to its speed at atmospheric pressure.
Materials and methods
Bottom sediment sample area—study area
The solid phase, i.e., top 5 cm of bottom sediments/sorbents, were collected in 2012 with a Van Veen grab from a depth of 10 m from the Port of Gdynia and in 2014 from a depth of 40 m from the area under influence of the Vistula River waters in the Gulf of Gdańsk (Fig. 1). The main types of harbor sediments are fluvioglacial sands, boulder clays, and silty clays (Dworniczak et al. 2017). The sediment types in the vicinity of the river (sample point 2) are fine sands and sandy silts. The sediments were air-dried, homogenized, and sieved through a 2-mm-diameter mesh sieve, and visible parts of plants, coal, bricks, and the like were removed. Before the experiments, performed in the years 2015–2016, sediments were kept in a freezer at − 21 °C.
Water sample area
The liquid phase, i.e., marine water, was sampled in 2015, from the Gulf of Gdańsk (Fig. 1) from a depth of 0.5 m using Teflon bottles and was aged for 6 months in room temperature for natural organic matter degradation. Typical salinity in the area of the Gulf of Gdansk is from 7.0 PSU around the Port of Gdynia to 3.0 PSU in the vicinity of the Vistula River mouth.
Before conducting the experiments, selected parameters of the bottom sediments (Table 1) and the water (Table 2) were determined after filtration through a GF/F filter.
Pentachlorophenol—sorptive
The solution of pentachlorophenol (PCP, CAS number 87-86-5) added to the marine water in the microcosm was prepared from standard solution (99.9%, Sigma-Aldrich, Poland) diluted with methanol to form a 5-μg-cm−3 stock solution. Development of microorganisms in the liquid phase was limited by adding sodium azide (NaN3) at a final concentration of 200 mg dm−3 (Chen et al. 2004).
Experimental setup at atmospheric pressure
The first part of the experiment was conducted at atmospheric pressure, at ambient temperature, and in the dark. The microcosm consisted of a 125-ml glass jar in which 20 g of the bottom sediments were placed and filled with marine water (Table 2), PCP, and NaN3 solutions in final volume of 40 ml. The control sample, done in triplicate, consisted of marine water, PCP, and NaN3 solution only.
Three sorbent types were prepared from the harbor sediments: Sandy (S)—grain size between 2.00 and 0.125 mm separated by mechanical shaking, Fine (F)—S sorbent with approximately 25% of grain size < 0.063 mm separated by mechanical shaking, and Ignited (I)—S sorbent ignited at 450 °C in a muffle furnace (Table 3). The microcosms with S, F, and I sorbents were done in four replicates. All microcosms were stabilized at atmospheric pressure for 1 day. Afterwards, 1 ml of PCP stock solution was added to liquid phase and the experiment was conducted for 8 and 24 h.
Bottom sediments from the Gulf of Gdańsk (Table 1) were used to prepare two sorbent types: Natural I (N I)—unmodified sediments from point 2 (Fig. 1) and Natural II (N II)—modified N I sorbent by adding approximately 25% of grain size < 0.063 mm separated by mechanical shaking, giving loss on ignition (LOI) of 3.4%. After initial exposure of N I (6 replicates) and N II (3 replicates) microcosms to 6000 hPa over 24 h, 1.6 ml of PCP stock solution was added to liquid phase and the sorption experiment at 1000 hPa was conducted for 8 h. In the following discussion, sorbent name is also name of microcosm.
Experimental setup at increased pressure
The experiment at 6000 hPa was conducted in the hyperbaric chamber of the Polish Naval Academy (PNA) in Gdynia (Fig. 2) keeping the same sorbent setup as for the experiment performed at atmospheric pressure. The required parameters were set via the control panel. The pressure was obtained by pumping atmospheric air into the chamber.
After each experiment, the overlying water was filtered through a combusted GF/F filter to glass vials fitted with Teflon caps. All the laboratory work was done at the Institute of Oceanography of the University of Gdańsk. The sorbents were dried at ambient temperature for 24 h and, after that, at 105 °C for 6 h. The water samples were kept at 7 °C for 12 h to 1 week and sorbents at − 21 °C for 1 week to 1 month.
In all the water samples and all the sediment samples, the fallowing measurements were taken: conductivity (n = 3)—with a Multi 3320 (WTW) and pH (n = 3)—with a pH meter (Elmetron). In the case of sorbents, measurements were done by pouring 30 ml of Milli-Q water onto 10 g of air-dried sediments and shaking for 1 h to reach stable reading. The concentration of Cl− anion (Mohr’s method, K2CrO4(aq)—indicator and 0.22 M AgNO3(aq)—titrator, n = 3) and sum of Ca2+ and Mg2+ cation concentration (EDTA method, Eriochrome Black T—indicator and 0.02 M EDTA—titrator, at pH = 10, n = 3) were determined in the overlying water from microcosms S, F, and I within 24 h after the end of the experiment. The samples from microcosms S, F, and I were used to assess the influence of pressure on water and sediment parameters. PCP was quantified in 30 water samples from microcosms N I and N II after exposure to 6000 hPa and in 28 water samples after exposure to 1000 hPa.
Organic matter determination
The analysis of the total organic carbon (TOC) concentration in the sediments was performed as described in Lewandowski et al. (2014). Sediment samples were oven dried (105 °C, 4 h) and acidified with 1.0 M HCl. Measurement of TOC was performed (n = 4) using a vario TOC cube analyzer (Elementar). The content of the total organic matter was determined by loss on ignition (LOI) method after burning dry sediments (n = 3) in muffle furnace at 550 °C for 8 h (Heiri et al. 2001). The precision of TOC analysis amounted to ± 0.03% for 1.61% TOC standard (n = 9) and ± 0.05% for 5.39% TOC standard (n = 13) and linear regression coefficient of TOC amount against the response of the detector amounted to r2 = 0.9998.
Analysis of PCP in water
The samples’ preparation by the solid-phase extraction method and analysis by high-performance liquid chromatography (HPLC) were done in accordance with the methodology validated by Kobusińska et al. (2014) and applied by Maciak et al. (2016). Purified extracts of water samples were injected on the chromatography column Hypersil GOLD thermostated at 25 °C and equipped with a UV-Vis detector. Chromatographic analysis was conducted for 25 min under gradient elution method using acetonitrile and Milli-Q water as the mobile phase.
Validation and quality parameters of PCP analysis
The recovery of PCP was assured based on external standard method and ranged from 90 to 113%. The precision expressed as the relative standard deviation (RSD) of the mean PCP concentration ranged from 1.8 to 4.7% and was set as 5%. Limit of quantification (LOQ) amounted to 15 ng g−1. The linearity of the calibration curve, based on 8 points ranged from 6.0 to 500.0 ng cm−3, with correlation coefficient of 0.999. The concentration of PCP in all control samples after 8 h of the experiment decreased by a mean of 8%.
Statistical analysis
The statistical analysis was carried out using non-parametric ANOVA Kruskal-Wallis test using add-in program Real Statistics (Zaiontz 2017) for the Excel 2010 software (Microsoft). The significance level was set as p < 0.05. The null hypothesis states that all result (values of PCP concentration and all physicochemical parameters) of all experiments for all samples will be statistically equal to each other.
Results
Parameters of the harbor sediments and the overlying water
There was a strong decrease in the content of organic matter (Table 4) after exposure of bottom sediments to 6000 hPa. Comparing the total organic carbon (TOC) concentration after the experiments at 6000 and 1000 hPa, the highest relative difference was found between I sorbents (50%), followed by S (21%) and F (15%) (Table 4). The TOC concentration in I, S, and F sorbents held at 6000 hPa for 24 h decreased by 85, 32, and 27%, respectively (Tables 3 and 4). In the case of ignited sediments held for 24 h at atmospheric pressure, the mean difference was 71% compared with the initial value. For sorbents S and F, the TOC concentration decreased by 17 and 18% respectively at 1000 hPa (Tables 3 and 4). The loss on ignition (LOI) method gave similar results. The LOI value of I, S, and F sorbents was lower after the hyperbaric exposure (Table 4).
The conductivity of sorbents held at atmospheric pressure did not differ among the sorbent groups and did not change after 8 h (number = 18, p value = 0.10) (Table 4). This parameter decreased by about 50% after 24 h of the experiment at 6000 hPa compared to results after 8-h exposure (Table 4).
The concentration of Cl−, Ca2+, and Mg2+ ions increased and pH decreased in the seawater of every microcosm at 1000 and 6000 hPa compared with the control samples. The highest concentration of Cl− ion was determined in the water from microcosm containing F sorbent and the lowest from microcosm with I sorbent. The 24-h exposure of microcosms to 6000 hPa resulted in lower chloride ion concentration in seawater compared to the results after 8 and 24 h at 1000 hPa. The highest increase of Ca2+ and Mg2+ concentration was noted in the overlying water from I microcosm (Table 5). The sum of these ions in the waters from microcosms exposed to the increased pressure was lower than in the waters held at 1000 hPa. The results of conductivity measurement confirmed the results of ion concentration analysis. The pH of water was slightly alkaline during experiment (Table 5).
Parameters of N I and N II sorbents
There was no observable influence of the pressure increased to 6000 hPa on the initial content of organic matter in N I and N II* sorbents (Tables 1 and 6 and *information in the text).
The conductivity of sediments from the Gulf of Gdańsk held at 1000 hPa decreased when compared to the initial value (Tables 1 and 6). After the hyperbaric exposure, value of this parameter for N II sorbent was higher than that for N II sorbent held at atmospheric pressure (Tables 1 and 6).
Concentration of PCP in N I and N II microcosms
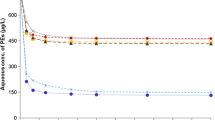
The sorption of PCP occurred at pH ≈ 8 (Table 7). On the basis of all results (n = 58), it was found that after 8-h exposure of N I (n = 19) and N II (n = 11) microcosms to 6000 hPa, the concentration of PCP in the overlying water was statistically significantly lower (p value = 0.001) when compared to N I (n = 19) and N II (n = 9) microcosms held at 1000 hPa (Fig. 3). Average difference between concentration of PCP in every microcosm held at higher pressure and every microcosm held at atmospheric pressure was 8%.
Discussion
Influence of the increased pressure on the sediment parameters
The sorption/desorption processes of a given substance depend on the characteristics of mineral structure, the type of organic matter, and the characteristic of water reservoir (LeBoeuf and Weber 1997; Chorover and Brusseau 2008; Niemirycz and Jankowska 2011). The changes of the physicochemical parameters of the sediments after exposure to the increased pressure probably also depended on these factors (Tables 4 and 5).
The initial LOI/TOC ratio was 2.10, 2.80, and 3.10 for I, S, and F sorbents, respectively (Table 3). Usually, the LOI to TOC conversion factor is in the range of 1.70–2.0 (Veres 2002; Rallakis et al. 2013). It is interesting to note that for F sediments after an 8-h hyperbaric exposure, this factor reached 2.90 and dropped significantly to 2.50 after 24 h (Table 4). The cause of this could be a slower process of stabilization (within 24 h) due to the F sediments containing an increased amount of the fine fraction (Φ < 0.063 mm) from the Port of Gdynia. Taking into account all the parameters of the bottom sediments, the highest difference between 8- and 24-h experiments was noted for F sorbent after exposure to the increased pressure.
The possible cause of the decrease of LOI and TOC values of S, F, and I sorbents after the first part of experiment, carried out at atmospheric pressure, could be the method of sediment preparation by initial air drying and rewetting. During such a procedure, the hydrogen bonds in the organic matter are broken. That leads to the destabilization of some structures of the organic matter and to the stronger dissolution of matter contained in the sediment (Birch 1959; Raveh and Avnimeleh 1978). The storage time (3 years) could also exert its effect on the harbor sediments. After the experiment with sorbents prepared using the sediments from the Gulf of Gdańsk, organic matter content was not affected even by the hyperbaric exposure (Table 6).
Beside the type of bottom sediments, the depth and environment from which sediments were sampled also seem to be important. The material for S, F, and I sorbents was silty sediments, originating from a depth of 10 m in the Port of Gdynia, where hydrostatic pressure is five times lower than that during experiment. Moreover, sediments in this disturbed benthic environment are subjected to regular intensive mixing by ships. Probably, this is why organic matter decreased even after atmospheric exposure. The material for N I and N II sorbents was sandy sediments, collected from less polluted environment of the Gulf of Gdańsk. The sampling depth was 40 m where pressure was 5000 hPa, close to that in the experiment, resulting in stable organic matter under applied conditions.
The increase of overall pressure could lead to increase in the solubility of minerals, allowing the water to reach the saturation point faster, which leads to the process of crystallization (Stoch 1974). Probably, this explains why conductivity of the harbor sediments was lower after experiment in the hyperbaric chamber (Table 4).
The hyperbaric exposure of the harbor sediments could be referred to the routine dredging works carried out in ports to deepen the bottom channels. According to the guidelines (HELCOM Guidelines 2007), the dredged uncontaminated material of appropriate diameter can be disposed in a selected area in the sea (dumping site), often at a different depth to the depth at which dredging works took place. In the case of the Port of Gdynia, where samples of sediments where collected, dumping site is located at a depth of 20–50 m (Michałek et al. 2014), where the total pressure reaches 3000 to 6000 hPa. Considering this, it is possible that observed changes of S, F, and I sorbent parameters are occurring in the natural environment. The results of this study can be taken into consideration by other researchers examining the areas of the dumping sites.
Influence of the increased pressure on the water parameters
The phenomenon of lowering Cl− ion concentration due to the influence of pressure force was observed in the first works describing extraction of the interstitial waters from sediments (Shishkina 1972; Lindström and Bloomquist 1980). The method involved using pressure 100 times greater than in the present work. Other researchers (Parashiva-Murthy and Perrel 1972), which applied pressure (6895 hPa) close to the one which was simulated inside the hyperbaric chamber, observed lowering of Ca2+ ions and increase of Mg2+ ions after squeezing the pore waters. Ions of Mg2+, K+, and also Fe2+ are the most mobile (Stoch 1974). Radke et al. (2012) revealed that the exposure of bottom sediments from the Port of Gdynia to the temperatures between 400 and 600 °C resulted in decarbonization of very fine-grained matter with magnesium carbonate. This can be the reason why the highest concentration of Ca2+ and Mg2+ was found in the water overlying the ignited harbor sediments. The dissolving of the organic matter and the hydronium ion transfer from minerals surface (Stoch 1974) could have an impact of lowering the water pH (Table 5).
The changes of the physicochemical parameters of the sediments and the water, which affect sorption, should not take place during such an experiment. This indicates that the initial hyperbaric exposure (before experiment) of microcosms could be necessary.
Influence of hydrostatic pressure on the sorption process
Hydrostatic pressure is presented in the scientific literature as one of the parameters which may, among others, influence the sorption/desorption processes (Grossl et al. 1997; Bigus et al. 2014) or increase/decrease growth of bacteria population (Somero 1992; Schedler et al. 2014; Scoma et al. 2016). However, there are not many laboratory studies examining the sorption of persistent organic pollutants (POPs) at a pressure higher than atmospheric. The pressure at bottom of the sea, in conditions of limited depth, may contain, in addition to the hydrostatic component, also hydrodynamic component of an oscillatory nature due to the presence of wind waves (Cieślikiewicz and Badur 2013). We estimate, however, that at a depth of 50 m, even in conditions of high winds, the hydrodynamic part of pressure varies within ± 2% of atmospheric pressure, i.e., ± 20 hPa. With the assumed pressure of 6000 hPa, this is a negligible force that we ignore in this work.
The pH is an important factor in the sorption and dissolution processes of PCP. The pH ≈ 8 of the overlying water means that PCP existed in an anionic form, which results in reduced hydrophobicity and limited sorption/desorption efficiency in humus or sediments (DiVincenzo and Sparks 2001; Site 2001). In laboratory studies concerning partitioning of PCP between solid and liquid phases, an 8-h period was sufficient to reach an equilibrium concentration (Site 2001; Chen et al. 2004; Subramanian et al. 2010; Liping et al. 2015). The influence of simulated hydrostatic pressure on the physicochemical parameters of N I and N II sorbents and its overlying water was minor or not significant (Tables 6 and 7) compared to S, F, and I sorbents (Tables 4 and 5). Based on the available data, it was assumed that the sorption process of PCP was occurring in stable conditions. The PCP concentrations in the overlying water of the microcosms containing N I and N II sorbents exposed in the experiment to the same pressure were compared (Fig. 3). The amount of fine fraction (1 or 25%) and organic matter (0.60 and 2.95% after the experiment) did not significantly affected the sorption process in the present study in accordance with findings of other papers (DiVincenzo and Sparks 2001; D’Angelo and Reddy 2003). Considering the specific nature of the harbor bottom sediments, we can determine that the hydrostatic pressure at 50 m appears to be inconsequential in the sorption process of PCP (Fig. 3).
According to the mass balance concept, the amount of substance that has entered the reservoir is equal to the amount that has left the reservoir and been accumulated in it. If the sorption process in bottom sediments is affected by hydrostatic pressure, higher than that in the experiment, it must also change with depth. In situation where influence of water and sediment parameters (Bigus et al. 2014) is negligible, for some of the substances, it may be easier to leave the reservoir or stay longer in it at different depths.
Conclusions
Hydrostatic pressure is an important, but scarcely studied parameter in the persistent organic pollutant sorption and desorption processes. The conducted study revealed complexity of all reactions occurring in the microcosms during the experiments. The pressure of 6000 hPa, corresponding to the depth of 50 m, had minor influence on the sorption of pentachlorophenol in the marine bottom sediments. No increase in PCP sorption in marine sediments is disadvantageous, since PCP and related compounds are then dissociated and/or suspended in the water column. Thus, they remain bioavailable for longer and can be absorbed by organisms at different trophic chain levels resulting in bioaccumulation and biomagnification. It is a subject of particular concern as the studied coastal waters represent a frequently visited recreation, seaside area.
The simulated pressure of 6000 hPa is relatively low in relation to the possible hydrostatic pressure that can be noted on the bottom of the deeper seas and oceans. Thus, the results presented in this study can provide only some new insight into the importance of hydrostatic pressure in the sorption/desorption processes, which occur on the seabed of the ocean systems.
References
Bigus P, Tobiszewski M, Namieśnik J (2014) Historical records of organic pollutants in sediment cores. Mar Pollut Bull 78(1–2):26–42
Birch HF (1959) Further observations on humus decomposition and nitrification. Plant Soil 10:9–31
Chen YX, Chen HL, Xu YT, Shen MW (2004) Irreversible sorption of pentachlorophenol to sediment: experimental observations. Environ Int. https://doi.org/10.1016/S0160-4120(03)00145-4
Chorover J, Brusseau ML (2008) Kinetics of sorption-desorption. In: Brantley S, Kubicki J, White A (eds) Kinetics of water-rock interaction. Springer, New York, pp 109–149
Cieślikiewicz W, Badur J (2013) Modelling of wind wave pressure and free-surface elevation using system identification. J Korean Soc Coast Ocean Eng 25(6):422–432
D’Angelo E, Reddy KR (2003) Effect of aerobic and anaerobic conditions on chlorophenol sorption in wetland soils. Soil Sci Soc Am J 67:787–794
DiVincenzo JP, Sparks DL (2001) Sorption of the neutral and charged forms of pentachlorophenol on soil: evidence for different mechanisms. Arch Environ Con Tox. https://doi.org/10.1007/s002440010196
Dworniczak J, Wróblewski R, Rudowski S, Sitkiewicz P, Bieszk M (2017) Bottom structure of selected basins in the Port of Gdynia. BMI. https://doi.org/10.5604/12307424.1223968
Grossl PR, Eick M, Sparks DL, Goldberg S, Ainsworth CC (1997) Arsenate and chromate retention mechanisms on goethite. 2. Kinetic evaluation using a pressure-jump relaxation technique. Environ Sci Technol 31:321–326
Hedman J, Tocca JS, Gunnarsson JS (2009) Remobilization of polychlorinated biphenyl from the Baltic Sea sediment: comparing the roles of bioturbation and physical resuspension. Environ Toxicol Chem 28(11):2241–2249
Hedman JE, Bradshaw C, Thorsson MH, Gilek M, Gunnarsson JS (2008) Fate of contaminants in the Baltic Sea sediments: role of bioturbation and settling organic matter. Mar Ecol-Prog Ser. https://doi.org/10.3354/meps07218
Heiri O, Lotter AF, Lemcke G (2001) Loss-on-ignition as a method for estimating organic and carbonate content in sediments: reproducibility and comparability of results. J Paleolimnol. https://doi.org/10.1023/A:1008119611481
HELCOM (2007) Guidelines for the disposal of dredged material at sea. Helsinki
Kobusińska M, Skauradszun M, Niemirycz E (2014) Factors determining the accumulation of pentachlorophenol—a precursor of dioxins in bottom sediments of the Gulf of Gdańsk (Baltic Sea). Oceanol Hydrobiol St. https://doi.org/10.2478/S13545-014-0128-9
LeBoeuf EJ, Weber WJ (1997) A distributed reactivity model for sorption by soils and sediments: 8. Sorbent organic domains: discovery of a humic acid glass transition and an argument for a polymer-based model. Environ Sci Technol 31(6):1687–1702
León-Santiesteban HH, Wrobel K, García LA, Revah S, Tomasini A (2014) Pentachlorophenol sorption by Rhizopus oryzae ENHE: pH and temperature effects. Water Air Soil Poll 225:1947
Lewandowski K, Witt M, Kobusińska M, Niemirycz E (2014) Polychlorinated dibenzo-p-dioxins (PCDDs), polychlorinated dibenzofurans (PCDFs) and pentachlorophenol (PCP) in bottom sediments of the Port of Gdańsk. Oceanol Hydrobiol St. https://doi.org/10.2478/s13545-014-0146-7
Lindström MCH, Bloomquist RG (1980) Influence of squeezing pressure on pore water composition of unconsolidated Baltic Sea sediments. In: Lindström MCH (ed) The formation of iron constituents during early diagenesis: in situ studies of a Baltic Sea sediment-water interface. Department of Geological Sciences, Stockholm
Liping L, Defu L, Huanyu C, Fang C, Yunfeng H, Guangming T (2015) The change of organic matter in sewage sludge composting and its influence on the adsorption of pentachlorophenol (PCP). Environ Sci Pollut Res. https://doi.org/10.1007/s11356-014-3756-0
Maciak J, Lewandowski K, Niemirycz E (2016) The migration of pentachlorophenol (PCP) in artificial and natural sediments of the Puck Bay area. Oceanol Hydrobiol St 45(3):368–376
Michałek M, Osowiecki A, Błeńska M, Smolarz K (2014) Report on the assessment of ecological risk at the dumping sites. Application of ecosystem principles for the location and management of offshore dumping sites in SE Baltic Region (ECODUMP). Maritime Institute in Gdańsk, Gdańsk
Niemirycz E, Jankowska D (2011) Concentrations and profiles of PCDD/Fs in sediments of major Polish rivers and the Gdansk Basin–Baltic Sea. Chemosphere 85:525–532
Olsen LM, Hernández KL, Ardelan MV, Iriarte JL, Bizsel KC, Olsen Y (2017) Responses in bacterial community structure to waste nutrients from aquaculture: an in situ microcosm experiment in a Chilean fjord. Aquacult Environ Interact https://doi.org/10.3354/aei00212
Parashiva-Murthy AS, Perrel RE (1972) Distribution of major cations in estuarine sediments. Clay Clay Miner 21:161–129
Puzyn T, Suzuki N, Haranczyk M (2008) How do the partitioning properties of polyhalogenated POPs change when chlorine is replaced with bromine? Environ Sci Technol. https://doi.org/10.1021/es8002348
Radke B, Wasik A, Jewell LL, Piketh S, Pączek U, Gałuszka A, Namieśnik J (2012) Seasonal changes in organotin compounds in water and sediment samples from the semi-closed Port of Gdynia. Sci Total Environ 441:57–66
Rallakis D, Siavalas G, Oskay RG, Tsimiklis D, Christanis K (2013) Maturity of dispersed organic matter in bituminous formations of the Ionian zone (Epirus region, NW Greece). Proceedings of the 13th International Congress, Chania
Raveh A, Avnimeleh Y (1978) The effect of drying on the colloidal properties and stability of humic compounds. Plant Soil 50:545–552
Schedler M, Hiessl R, Juárez AGV, Gust G, Müller R (2014) Effect of high pressure on hydrocarbon-degrading bacteria. AMB Express 4(77):1–7
Scoma A, Barbato M, Hernandez-Sanabria E, Mapelli F, Daffonchio D, Borin S, Boon N (2016) Microbial oil-degradation under mild hydrostatic pressure (10 MPa): which pathways are impacted in piezosensitive hydrocarbonoclastic bacteria? Sci Rep. https://doi.org/10.1038/srep23526
Shishkina OV (1972) Geochemistry of marine interstitial waters. Nauka, Moscow (in Russian)
Site DA (2001) Factors affecting sorption of organic compounds in natural sorbent/water systems and sorption coefficients for selected pollutants. A Review J Phys Chem Ref Data 30(1):187–439
Somero GN (1992) Adaptations to high hydrostatic pressure. Annu Rev Physiol 54:557–577
Soubaneh YD, Gagné J-P, Lebeuf M, Nikiforov V, Gouteux B, Osman AM (2015) Sorption and competition of two persistent organic pesticides onto marine sediments: relevance to their distribution in aquatic system. Chemosphere https://doi.org/10.1016/j.chemosphere.2015.02.039
Stoch L (1974) Clay minerals. Geological Press, Warsaw (in Polish)
Subramanian B, Namboodiri V, Khodadoust AP, Dionysiou DD (2010) Extraction of pentachlorophenol from soils using environmentally benign lactic acid solutions. J Hazard Mater 174:263–269
The Stockholm Convention on Persistent Organic Pollutants (2015) The new POPs under the Stockholm Convention: listing of pentachlorophenol and its salts and esters. https://chm.pops.int/TheConvention/ThePOPs/TheNewPOPs/tabid/2511/Defaultaspx. Accessed 19 January 2017
U. S. Department of Health and Human Services (2014) Report on carcinogens Monograph on pentachlorophenol and by-products of its synthesis. https://ntp.niehs.nih.gov/ntp/roc/thirteenth/monographs_final/pentachlorophenol_508.pdf. Accessed 8 January 2017
Veres DS (2002) A comparative study between loss on ignition and total carbon analysis on minerogenic sediments. Geology 47(1):171–182
Witt M (2015) Accumulation of persistent organic pollutants in the bottom sediments of the Baltic Sea and impact of atmospheric deposition on this process. University of Gdańsk, Dissertation
Zaiontz C (2017) Real Statistics using Excel. wwwreal-statisticscom Accessed (10 January 2016)
Acknowledgements
The authors would like to thank to Commander Adam Olejnik and Commander Arkadiusz Woźniak and all staff of the Department of Underwater Works Technology of Polish Naval Academy in Gdynia for a great cooperation during the study.
Funding
This research was partially funded from the resources of the research project for Ph.D. students and young scientists at the University of Gdańsk, project no. 538–G235–B879–15-1M and statutory activities of the University of Gdańsk.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lewandowski, K.K., Cieślikiewicz, W., Kobusińska, M.E. et al. Sorption of pentachlorophenol (PCP) in the marine bottom sediments—batch sorption experiment at varying pressure. Environ Sci Pollut Res 25, 10799–10807 (2018). https://doi.org/10.1007/s11356-017-1076-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-1076-x