Abstract
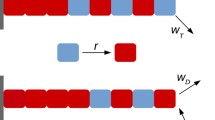
Simulations of microtubule oscillations have been obtained by a kinetic model including nucleation of microtubules, elongation by addition of GTP-loaded tubulin dimers, disassembly into oligomers, and dissolution of oligomers followed by nucleotide exchange at the free dimers. Dynamic instability is described by the on and off rates for dimer association in the growth phase, the rate of rapid shortening, and the transition rates for catastrophe and rescue. The latter are assumed to be completely determined by the current state of the system (“short cap hypothesis”). Microtubule oscillations and normal polymerizations measured by time-resolved X-ray scattering were used to test the model. The model is able to produce oscillations without further assumptions. However, in order to obtain good fits to the experimental data one requires an additional mechanism which prevents rapid desynchronization of the microtubules. One of several possible mechanisms that will be discussed is the destabilization of microtubules by the products of disassembly.
Similar content being viewed by others
Abbreviations
- MT(s):
-
microtubule(s)
- G-MT/S-MT:
-
microtubule in the state of growth/shortening
- GTP:
-
guanosine 5′-triphosphate
- GDP:
-
guanosine 5′-diphosphate
- TU · GDP/TU · GTP:
-
tubulin dimer with GDP/GTP bound to the exchangeable nucleotide binding site
- MAP(s):
-
microtubule-associated protein(s)
- PC:
-
tubulin phosphocellulose-purified tubulin
- PIPES:
-
piperazine-1,4-bis(2-ethane sulfonic acid)
- DDT:
-
dithiothreitol
- EGTA:
-
ethylene glycol-O,O′-bis(2-amino ethyl ether)-N,N,N′,N′-tetraacetic acid
References
Bayley PM, Manser EJ (1985) Assembly of microtubules from nucleotide-depleted tubulin. Nature 318:683–685
Bayley PM, Martin SR (1986) Inhibition of microtubule elongation by GDP. Biochem Biophys Res Commun 137:351–358
Bayley PM, Schilstra MJ, Martin SR (1989) A simple formulation of microtubule dynamics: quantitative implications of the dynamic instability of microtubule populations in vivo and in vitro. J Cell Sci 93:241–254
Bayley P, Schilstra M, Martin S (1989a) A lateral cap model of microtubule dynamic instability. FEBS Lett 259:181–184
Bayley PM, Schilstra MJ, Martin SR (1990) Microtubule dynamic instability: numerical simulation of microtubule transition properties using a lateral cap model. J Cell Sci 95:33–48
Boulin C, Kempf R, Koch M, McLaughlin S (1986) Data appraisal, evaluation and display for synchrotron radiation experiments: hardware and software. Nucl Instrum Methods A 249:399–407
Caplow M (1992) Microtubule dynamics. Curr Opinion Cell Biol 4:58–65
Carlier M-F, Pantaloni D (1981) Kinetic analysis of guanosine 5′triphosphate hydrolysis associated with tubulin polymerization. Biochemistry 20:1918–1924
Carlier M-F, Melki R, Pantaloni D, Hill TL, Chen Y (1987) Synchronous oscillations in microtubule polymerization. Proc Natl Acad Sci, USA 84:5257–5261
Carlier MF, Didry D, Melki R, Chabre M, Pantaloni D (1988) Stabilization of microtubules by inorganic phosphate and its structural analogues, the fluoride complexes of aluminum and beryllium. Biochemistry 27:3555–3559
Cassimeris L, Pryer NK, Salmon ED (1988) Real-time observation of microtubule dynamic instability in living cells. J Cell Biol 107:2223–2231
Chen Y, Hill TK (1985) Monte Carlo study of the GTP cap in a five-start helix model of a microtubule. Proc Natl Acad Sci, USA 82:1131–1135
Chen Y, Hill TL (1987) Theoretical studies on oscillations in microtubule polymerization. Proc Natl Acad Sci, USA 84:8419–8423
Chretien D, Metoz F, Verde F, Karsenti E, Wade RH (1992) Lattice defects in microtubules: protofilament numbers vary within individual microtubules. J Cell Biol 117:1031–1040
Cone M, Lombillo VA, McIntosh JR (1991) Microtubule depolymerization promotes particle and chromosome movement in vitro. J Cell Biol 112:1165–1175
Drechsel DN, Hyman AA, Cobb MH, Kirschner MW (1992) Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Mol Biol Cell 3:1141–1154
Engelborghs Y, Eccleston J (1982) Fluorescence stopped-flow study of the binding of S6-GTP to tubulin. FEBS Lett 141:78–81
Erickson HP, O'Brien ET (1992) Microtubule dynamic instability and GTP hydrolysis. Annu Rev Biophys Biomol Struct 21:145–166
Gaskin F, Cantor CR, Shelanski MI (1974) Turbidimetric studies of the in vitro assembly and disassembly of porcine neurotubules. J Mol Biol 89:737–758
Gildersleeve RF, Cross AR, Cullen KE, Fagen AP, Williams RC (1992) Microtubules grow and shorten at intrinsically variable rates. J Biol Chem 267:7995–8006
Hamel E, Batra J, Lin C (1986) Direct incorporation of guanosine 5′-diphosphate into microtubules without guanosine 5′-triphosphate hydrolysis. Biochemistry 25:7054–7062
Hill TL, Chen Y (1984) Phase changes at the end of a microtubule with a GTP cap. Proc Natl Acad Sci, USA 81:5772–5776
Hitt AL, Cross AR, Williams RC (1990) Microtubule solutions display nematic liquid-crystalline structure. J Biol Chem 265:1639–1647
Horio T, Hotani H (1986) Visualization of the dynamic instability of individual microtubules by dark-field microscopy. Nature 321:605–607
Howard WD, Timasheff SN (1986) GDP state of tubulin: stabilization of double rings. Biochemistry 25:8292–8300
Hyman AA, Salser S, Drechsel DN, Unwin N, Mitchison TJ (1992) Role of GTP hydrolysis in microtubule dynamics: information from a slowly hydrolysable analogue, GMPCPP. Mol Biol Cell 3:1155–1167
Koch MHJ, Bordas J (1983) X-ray diffraction and scattering on disordered systems using synchrotron radiation. Nucl Instrum Methods 208:461–469
Koshland DE, Mitchison TJ, Kirschner MW (1988) Polewards chromosome movement driven by microtubule depolymerization in vitro. Nature 331:499–504
Kristofferson D, Karr TL, Purich DL (1980) Dynamics of linear protein polymer disassembly. J Biol Chem 255:8567–8572
Lange G, Mandelkow E-M, Jagla A, Mandelkow E (1988) Tubulin oligomers and microtubule oscillations — antagonistic role of microtubule stabilizers and destabilizers. Eur J Biochem 178:61–69
Lin CM, Hamel E (1987) Interrelationships of tubulin-GDP and tubulin-GTP in microtubule assembly. Biochemistry 26:7173–7182
Mandelkow E, Mandelkow EM, Bordas J (1983) Synchrotron radiation as a tool for studying microtubule self-assembly. TIBS 8:374–377
Mandelkow E-M, Mandelkow E (1992) Microtubule oscillations. Cell Motility Cytoskeleton 22:235–244
Mandelkow E-M, Harmsen A, Mandelkow E, Bordas J (1980) X-ray kinetic studies of microtubule assembly using synchrotron radiation. Nature 287:595–599
Mandelkow E-M, Herrmann M, Rühl U (1985) Tubulin domains probed by subunit-specific antibodies and limited proteolysis. J Mol Biol 185:311–327
Mandelkow E-M, Lange G, Jagla A, Spann U, Mandelkow E (1988) Dynamics of the microtubule oscillator: role of nucleotides and tubulin-MAP interactions. EMBO J 7:357–365
Mandelkow E-M, Mandelkow E,Milligan RA (1991) Microtubule dynamics and microtubule caps: a time-resolved cryo-electron microscopy study. J Cell Biol 114:977–991
Martin SR, Bayley PM (1987) Effects of GDP on microtubules at steady state. Biophys Chem 27:67–76
Martin SR, Schilstra MJ, Bayley PM (1991) Opposite-end behaviour of dynamic microtubules. Biochim Biophys Acta 1073:555–561
Martin SR, Schilstra MJ, Bayley PM (1993) Dynamic instability of microtubules: Monte Carlo simulation and application to different types of microtubule lattice. Biophys J 65:578–596
Marx A, Jagla A, Mandelkow E (1990) Microtubule assembly and oscillations induced by flash photolysis of caged-GTP. Fur Biophys J 19:1–9
Melki R, Carlier M-F, Pantaloni D (1988) Oscillations in microtubule polymerization: the rate of GTP regeneration on tubulin controls the period. EMBO J 7:2653–2659
Melki R, Carlier M-F, Pantaloni D, Timasheff SN (1989) Cold depolymerization of microtubules to double rings: geometric stabilization of assemblies. Biochemistry 28:9143–9152
Mitchison T, Kirschner M (1984) Dynamic instability of microtubule growth. Nature 312:237–242
Obermann H, Mandelkow E-M, Lange G, Mandelkow E (1990) Microtubule oscillations: role of nucleation and microtubule number concentration. J Biol Chem 265:4382–4388
Obermann-Pleß H (1992) Zusammenhänge zwischen Nukleation, dynamischer Instabilität and Oszillationen von Mikrotubuli. Doctoral thesis, Hamburg
O'Brien ET, Voter WA, and Erickson HP (1987) GTP hydrolysis during microtubule assembly. Biochemistry 26:4148–4156
O'Brien ET, Salmon ED, Walker RA, Erickson HP (1990) Effects of magnesium on the dynamic instability of individual microtubules. Biochemistry 29:6648–6656
Pirollet F, Job D, Margolis RL, Garel J-R (1987) An oscillatory mode for microtubule assembly. EMBO J 6:3247–3252
Press WH, Flannery BP, Teukolsky SA, Vetterling WT (1988) Numerical recipes. Cambridge University Press, Cambridge
Pryer NK, Walker RA, Skeen VP, Bourns BD, Soboeiro MF, Salmon ED (1992) Brain microtubule-associated proteins modulate microtubule dynamic instability in vitro. J Cell Sci 103:965–976
Renner W, Mandelkow E-M, Mandelkow E, Bordas J (1983) Self-assembly of microtubule protein studied by time-resolved X-ray scattering using temperature jump and stopped flow. Nucl Instrum Methods 208:535–540
Rothwell SW, Grasser WA, Baker HN, Murphy DB (1987) The relative contributions of polymer annealing and subunit exchange to microtubule dynamics in vitro. J Cell Biol 105:863–874
Sammak PJ, Borisy GG (1988) Direct observation of microtubule dynamics in living cells. Nature 332:724–726
Schilstra MJ, Martin SR, Bayley PM (1987) On the relationship between nucleotide hydrolysis and microtubule assembly: Studies with a GTP-regenerating system. Biochem Biophys Res Commun 147:588–595
Schulze E, Kirschner M (1988) New features of microtubule behavior observed in vivo. Nature 334:356–359
Smith P, Krohn I, Hermanson G, Malla A, Gartner F, Provenzano M, Fujimoto E, Goeke N, Olson B, Klenk D (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Somers M, Engelborghs Y (1990) Kinetics of the spontaneous organization of microtubules in solution. Fur Biophys J 18:239–244
Spann U, Renner W, Mandelkow E-M, Bordas J, Mandelkow E (1987) Tubulin oligomers and microtubule assembly studied by time-resolved X-ray scattering: separation of prenucleation and nucleation events. Biochemistry 26:1123–1132
Stewart RJ, Farell KW, Wilson L (1990) Role of GTP hydrolysis in microtubule polymerization: evidence for a coupled hydrolysis mechanism. Biochemistry 29:6489–6498
Tabony J, Job D (1990) Spatial structures in microtubular solutions requiring a sustained energy source. Nature 346:448–451
Tabony J, Job D (1992) Gravitational symmetry-breaking in microtubular dissipative structures. Proc Natl Acad Sci, USA 89:6948–6952
Trinczek B, Marx A, Mandelkow E-M, Murphy DB, Mandelkow E (1993) Dynamics of microtubules from erythrocyte marginal bands. Mol Biol Cell 4:323–335
Voter WA, Erickson HP (1984) The kinetics of microtubule assembly: evidence for a two-stage nucleation mechanism. J Biol Chem 259:10430–10438
Voter WA, O'Brien ET, Erickson HP (1991) Dilution-induced disassembly of microtubules: relation to dynamic instability and the GTP cap. Cell Motility & Cytoskeleton 18:55–62
Wade RH, Pirollet F, Margolis RL, Garel J-R, Job D (1989) Monotonic versus oscillating microtubule assembly: a cryo-electron microscope study. Biol Cell 65:37–44
Walker RA, O'Brien ET, Pryer NK, Soboeiro M, Voter WA, Erickson HP, Salmon ED (1988) Dynamic instability of individual microtubules analysed by video light microscopy: rate constants and transition frequencies. J Cell Biol 107:1437–1448
Walker RA, Pryer NK, Salmon ED (1991) Dilution of individual microtubules observed in real-time in vitro: evidence that cap size is small and independent of elongation rate. J Cell Biol 114:73–81
Zeeberg B, Cheek J, Caplow M (1980) Exchange of tubulin dimer into rings in microtubule assembly-disassembly. Biochemistry 19:5078–5086
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Marx, A., Mandelkow, E. A model of microtubule oscillations. Eur Biophys J 22, 405–421 (1994). https://doi.org/10.1007/BF00180162
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00180162