Summary
2-hydroxy-4-trifluoromethylbenzoic acid (HTB) is the main active metabolite of the platelet anti-aggregant drug triflusal. Its binding to plasma proteins of rats and healthy volunteers in vitro and in vivo has been studied.
Rats were given a single oral dose of 50 mg·kg−1 triflusal and the healthy volunteers received 300 mg as a single oral dose or a multiple dose regimen of 600 mg every 24 h and 300 mg every 8 h, both for 13 days. Protein-free HTB was obtained by ultrafiltration. Unbound and total HTB concentrations were determined by HPLC.
HTB was primarily bound to albumin in plasma. The Scatchard plots suggested two types of binding sites for HTB on the albumin molecule. In rats, the binding constants (K=intrinsic affinity constant, n=number of binding sites) were K1=1.4×105 l·mol−1, n1=1.23, and K2=4.1×103 l·mol−1 and n2=3.77. The mean plasma concentration in rats after oral administration was 185 (37) μg·ml−1 (protein-free HTB: 2.44 (0.77)%). The binding constants in human plasma were K1=4.7×105 l·mol−1, n1=1.93, K2=4.3 l·mol−1 and n2=4.28.
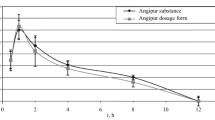
The plasma HTB concentration in man (n=8) was 35 μg·ml−1 (Cmax) after a single oral dose of triflusal 300 mg, 172.96 μg·ml−1 (Cmax·ss) during the multiple dosage regimen of 300 mg every 8 h, and 131 μg·ml−1 (Cmax·ss) during the multiple oral dose regimen of 600 mg every 24 h. Unbound HTB ranged from 0.27 to 0.43%, depending on dose. HTB had high affinity for plasma albumin, which was not saturable after therapeutic doses. It showed linear elimination.
Similar content being viewed by others
References
Albors M, Castellarnau C, Vila L, Solà J, Rutllant M (1987) Inhibition of thromboxane production and platelet function by triflusal in healthy volunteers. Rev Farmacol Clin Exp 4: 11–16
Borga O, Odar-Cederlof I, Ringberger V, Norlin A (1976) Protein binding of salicylate in uremic and normal plasma. Clin Pharmacol Ther 20: 464–475
Davison C, Smith P (1961) The binding of salicylic acid and related substances to purified proteins. J Pharmacol Exp Ther 133: 161–173
De la Cruz J, Pavia J, Bellido J, González M, Sánchez de la Cuesta F (1988) Platelet antiaggregatory effect of triflusal in human whole blood. Methods Find Exp Clin Pharmacol 10: 273–277
Demestre I, Sagarra R, Palacios G, Roser R (1989) A study of reference parameters in the CFY (remote Sprague-Dawley) rat. Methods Find Exp Clin Pharmacol 11: 101–110
Domínguez M, Vacas H, Saez Y, Olabarria I, Velasco A, Iriarte J, Forn J (1985) Effects of triflusal in patients with prosthetic heart valves. Clin Ther 7: 357–360
Gala F, García P, Gómez J, García Morato P (1986) Profilaxis de la trombosis venosa profunda en traumatizados. Biol Clin Hematol 8: 89
García-Rafanell J, Morell J (1977) L'effect inhibiteur de l'acide 2-acetoxy-4-trifluoromethylbenzoïque (triflusal) et de l'aspirine sur l'agrégation des plaquettes chez l'homme et le rat. Thérapie 32: 337–344
Guiteras P, Altimiras J, Arís A, Augé J, Bassons T, Bonal J, Caralps J, Castellarnau C, Crexells C, Masotti M, Oriol M, Padró J, Rutllant M (1989) Prevention of aortocoronary vein-graft attrition with low dose aspirin and triflusal both associated with dipyridamole: a randomized double-blind, placebo controlled trial. Eur Heart J 10: 159–167
Kramer E, Routh J (1973) The binding of salicyclic acid and acetylsalicylic acid to human serum albumin. Clin Biochem 6: 98–105
Levy G (1976) Effect of plasma protein binding of drugs on duration and intensity of pharmacological activity. J Pharm Sci 65: 1264–1265
Lin J, Ulm E, Duggan D (1986) Possible mechanism for reduced plasma clearance of diflunisal in rat experimental renal failure. J Pharmacol Exp Ther 238: 978–984
Martí R, Rocha E, Narvaiza M, Honorato J (1979) Prevention of postoperative deep vein thrombosis (DVT) by triflusal. Study of platelet aggregation, coagulation and DVT detection with 125I-fibrinogen. Curr Ther Res 25: 791–803
Metzler CM, Weiner DL (1985) PCNONLIN: User's guide (Vol. 1) Statistical Consultants, Lexington, KY
Nelder JA, Mead R (1965) A simplex method for function minimization. Comput J 7: 308–313
Oie S, Tozer TN (1979) Effects of altered plasma protein binding on apparent volume of distribution. J Pharm Sci 68: 1203–1205
Ramis J, Mis R, Forn J (1991a) Pharmacokinetics of triflusal and its main metabolite in rats and dogs. Eur J Drug Metab Pharmacokinet (in press)
Ramis J, Mis R, Forn J, Torrent J, Gorina E, Jane F (1991b) Pharmacokinetics of triflusal and its main metabolite HTB in healthy subjects following a single oral dose. Eur J Drug Metab Pharmacokinet (in press)
Rimbau V, López R, Fernández A, Forn J (1981) Estudio farmacocinético del triflusal (UR-1501). Arch Farmacol Toxicol 7: 11–16
Rogers HJ, Spector RG, Morrison PJ, Bradbrook ID (1981) Comparative steady state pharmacokinetic study of piroxicam and flurbiprofen in normal subjects. Eur J Rheumatol Inflamm 4: 303–308
Rutllant ML, Borell M, Felez J, Diaz JM, Vicente JM, García-Rafanell J (1977) Effect of triflusal (UR-1501) a potential antithrombotic agent, on blood coagulation and platelet aggregation in man. Curr Ther Res 22: 510–521
Sjoholm I, Ekman B, Kober A, Ljungstedt-Pahlman I, Seiving B (1979) Binding of drugs to human serum albumin: XI. The specificity of three binding sites as studied with albumin immobilized in microparticles. Mol Pharmacol 16: 767–777
Sudlow G, Birkett DJ, Wade DN (1975) The characterization of two specific binding sites on human serum albumin. Mol Pharmacol 11: 824–832
Tillement JP, Lhoste F, Giudicelli JF (1978) Diseases and drug protein binding. Clin Pharmacokinet 3: 144–154
Verbeeck R, Blackburn JL, Loewen GR (1983) Clinical pharmacokinetics of non-steroidal anti-inflammatory drugs. Clin Pharmacokinet 8: 297–331
Wosilait WD (1976) Theoretical analysis of the binding of salicylate by human serum albumin: The relationship between free and bound drug. Eur J Clin Pharmacol 9: 285–290
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mis, R., Ramis, J., Conte, L. et al. Binding of a metabolite of triflusal (2-hydroxy-4-trifluoromethylbenzoic acid) to serum proteins in rat and man. Eur J Clin Pharmacol 42, 175–179 (1992). https://doi.org/10.1007/BF00278480
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00278480