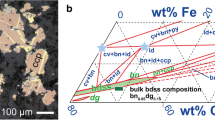
Abstract
Textural interpretation of ore-mineral assemblages, such as bornite-chalcopyrite (bn-cpy) intergrowths, should be based on definite experimentation. Appropriate exsolution and coarsening experiments were performed using sealed, evacuated, silicaglass tubes; synthetic bn-cpy solid solutions were annealed between 100° and 350°C for times of 20 min to 10 weeks. Early-formed textures develop through nucleation and growth and depend on the initial degree of supersaturation and the metal diffusivities. Final textures, due to additional growth and coarsening, are very sensitive to temperature and may serve as geothermometers. Mutual boundary textures which form above 250°C can originate by: (1) simultaneous precipitation; (2) exsolution during slow cooling from above the solvus; and (3) metamorphism to temperatures above about 250°C. Widmanstätten textures are not compatible with slow cooling, but indicate: (1) low-temperature replacement of bn; or (2) exsolution of cpy lamellae from anomalous bn heated to around 200°–250°C during mild metamorphism.
Similar content being viewed by others
References
Barton PB Jr (1970) Sulfide Petrology. MSA Spec Paper 3: 187–198
Barton PB Jr, Skinner BJ (1967) Sulfide Mineral Stabilities. In: Geochemistry of Hydrothermal Ore Deposits, Barnes H. L. (ed) Holt, Rinehart, Winston, New York pp 236–333
Barton PB Jr, Toulmin P III (1961) Some Mechanisms for Cooling Hydrothermal Fluids. USGS Prof Paper 424-D, 348–352
Birchenall CE (1973) Diffusion in Sulfides. In: Geochemical Transport and Kinetics, Giletti AW et al (ed) Carnegie Inst. Washington Publ. 634: 53–59
Brett PR (1962) Exsolution Textures and Rates in Solid Solutions Involving Bornite. Carnegie Inst Washington Yearb 61: 155–157
Brett PR (1963) The Cu-Fe-S System. Carnegie Inst Washington Yearb 62: 193–196
Brett PR (1964) Experimental Data from the System Cu-Fe-S and their Bearing on Exsolution Textures in Ores. Econ Geol 59: 1241–1269
Brett PR, Yund RA (1964) Sulfur-Rich Bornites. Am Min 49: 1084–1099
Cabri JL (1973) New Data on Phase Relations in the Cu-Fe-S System. Econ Geol 68: 443–454
Chen JH, Harvey VW (1975) Cation Self-Diffusion in Chalcopyrite and Pyrite. Metall Trans B 6: 331–339
Condit RH, Hobbins RR, Birchenall CE (1974) Self-Diffusion of Iron and Sulfur in Ferrous Sulfide. Oxid Met 8: 409–455
Durazzo A, Taylor LA (1978) Kinetic Factors in Bornite-Chalcopyrite Textural Developments. EOS Trans Am Geoph Union 59: 396
Dutrizac JE, MacDonald RJC, Ingraham TR (1970) The Kinetics of Dissolution of Bornite in Acidified Ferric Sulfate Solutions. Met Trans 1: 225–231
Edwards AB Textures of the Ore Minerals and their Significance. Melbourne: Australasian Inst of Mining and Met 1954
Frenzel G (1959) Idait und “blaubleibender Covellin”. Neues Jb Miner Abh 93: 87–132
von Gehlen K (1963) Anomaler Bornit und seine Umbildung zu Idait und “Chalkopyrit” in deszendenten Kupfererzen von Sommerkahl (Spessart). Fortschr Mineral 41: 163
Gruner JR (1929) Structural Reasons for Oriented Intergrowths in Some Minerals. Am Min 14: 227–237
Hawley JE (1962) The Sudbury Ores: Their Mineralogy and Origin. Can Min 7: 1–107
Hawley JE, Haw VA (1957) Intergrowths of Pentlandite and Pyrrhotite. Econ Geol 52: 132–139
Kullerud G (1971) Experimental Techniques in Dry Sulfide Research. In: Research Techniques for High Pressure and High Temperature. Ulmer GC (ed). Springer, Berlin 288–315
Kurtz AD, Averbach BL, Cohen M (1955) Self-Diffusion of Gold in Gold-Nickel Alloys. Acta Metall 3: 442–446
Lyon RJP (1959) Time Aspects of Geothermometry. Mining Eng 11: 1145–1151
MacLean WH, Cabri LJ, Gill JE (1972) Exsolution Products in Heated Chalcopyrite. Can J Earth Sci 9: 1305–1317
Martin JW, Doherty RD (1976) Stability of Microstructure in Metallic Systems. Cambridge Univ. Press, Cambridge
Morimoto N, Greig JW, Tunnel J (1960) Re-Examination of a Bornite from the Carn Brea Mine, Cornwall. Carnegie Inst Washington Yearb 59: 122–126
Newhouse WH (1928) The Microscopic Criteria of Replacement in the Opaque Ore Minerals. In: The Laboratory Investigation of Ores, Fairbanks EE (ed) McGraw-Hill, New York 147–161
Prouvost J (1960) Remarques sur la Composition et le Pouvoir Reflecteur de la Bornite (Cu5FeS4). Congr Nat Soc Savantes, Sect Sci, Dijon 1959 365–372
Ramdohr P (1969) The Ore Minerals and their Intergrowths. Pergamon Press, Oxford
Ray JR (1930) Sulfide Replacement of Ore Minerals. Econ Geol 25: 433–451
Roberts WMB (1963) The Low Temperature Synthesis in Aqueous Solution of Chalcopyrite and Bornite. Econ Geol 58: 52–61
Samal GI, Gilevich MP (1978) Self-Diffusion of Copper in Bornite and Chalcopyrite. Vestsi Akad Navuk BSSR, Ser Khim Navuk 1: 126–129
Schouten C (1934) Structures and Textures of Synthetic Replacements in “Open Space”. Econ Geol 29: 611–658
Schwartz GM (1931a) Integrowths of Bornite and Chalcopyrite. Econ Geol 26: 611–658
Schwartz GM (1931b) Textures due to Unmixing of Solid Solutions. Econ Geol 26: 739–763
Shewmon PG (1969) Transformations in Metals. Mc Graw-Hill, New York
Sillitoe RM, Clark AM (1969) Copper and Copper-Iron Sulfides as the Initial Products of Supergene Oxidation, Copiapo Mining District, Northern Chile. Am Min 54: 1684–1710
Sindeeva ND (1964) Mineralogy and Types of Deposits of Selenium and Tellurium, Interscience, New York
Sugaki A (1951a) Thermal Experiments on the Lattice Intergrowth of Chalcopyrite and Klaprothite in Bornite from the Obari Mine, Jpn Sci Rep Tohoku Univ, Ser III 4: 11–17
Sugaki A (1951b) Thermal Studies on the Lattice Intergrowths of Chalcopyrite in Bornite from the Akayama Mine, Jpn Sci Rep Tohoku Univ, Ser III 4: 19–28
Sugaki A (1955) Thermal Studies on the Lattice Intergrowths of Chalcopyrite in Bornite from the Jinmu Mine, Jpn Sci Rep Tohoku Univ, Ser III 5: 113–128
Sugaki A (1965) Studies on the Join Cu5FeS4-CuFeS2-x as Geothermometer. J Jpn Assoc Min Petr Econ Geol 53: 1–17
Taylor LA (1971) The Fe-S-O System: Oxidation of Pyrrhotite. Carnegie Inst Washington Yearb 70: 287–290
Tufar W (1967) Der Bornit von Trattenbach (Niederösterreich). Neues Jahrb Mineral Abh 106: 334–351
Van der Veen RW (1925) Mineragraphy of Ore Deposition. Naeff G, The Hague
Verhoeven JD (1975) Fundamentals of Physical Metallurgy. John Wiley & Sons, New York
Wandke A (1926) Molecular Migration and Mineral Transformation. Econ Geol 28: 166–171
Yund RA, Hall HT (1970) Kinetics and Mechanism of Pyrite Exsolution from Pyrrhotite. J Petr 11: 381–404
Yund RA, Kullerud G (1966) Thermal Stability of Assemblages in the Cu-Fe-S System. J Petr 7: 454–488
Yund RA, McCallister RH (1970) Kinetics and Mechanisms of Exsolution. Chem Geol 6: 5–30
Ziebold TO, Ogilvie RE (1963) Quantitative Analysis with the Electron Microanalyzer. Analyt Chem 35: 621–627
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Durazzo, A., Taylor, L.A. Experimental exsolution textures in the system bornite-chalcopyrite: Genetic implications concerning natural ores. Mineral. Deposita 17, 79–97 (1982). https://doi.org/10.1007/BF00206377
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00206377