Abstract
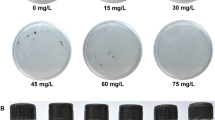
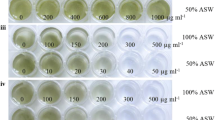
Isochrysis galbana and Isochrysis sp. are economically important microalgae from the division of haptophytes. Here, we report Agrobacterium-mediated stable DNA transfer into their nuclear genomes. Initial studies were performed to standardize co-cultivation media and determine the sensitivity of the microalgae to selective agents. Up to 1 mg/ml of the antibiotic hygromycin did not inhibit growth, whereas both the haptophytes bleached in artificial seawater (ASW) medium containing micromolar concentrations of the herbicide norflurazon. Co-cultivation of Isochrysis sp. and I. galbana with Agrobacterium tumefaciens strain LBA 4404 harboring the binary vector pCAMBIA 1380-pds-L504R yielded norflurazon-resistant (NR) colonies visible on selective plates after 20–30 days. pCAMBIA 1380-pds-L540R was constructed by cloning a mutated genomic phytoene desaturase (pds) gene from Haematococcus pluvialis as a selectable marker gene into the binary vector system pCAMBIA 1380. Co-cultivation of Isochrysis sp. with A. tumefaciens in ASW medium containing 200 μM of acetosyringone for 72 h produced the highest number of NR cells. For I. galbana, 100 μM of acetosyringone, ASW medium, and 48 h co-cultivation period appeared to be optimum co-cultivation parameters. The NR colonies kept their resistance phenotype for at least 24 months, even in the absence of selective pressure. The transfer of the pds gene in NR cells was shown by PCR amplification of the T-DNA sequences from the genomic DNA of NR cells and Southern blot analysis using T-DNA sequences as probes. The genetic manipulation described here will allow metabolic engineering and a better understanding of several biochemical pathways in the future.






Similar content being viewed by others
References
Ali S, Xianyin Z, Xue Q, Hassan MJ, Qian H (2007) Investigations for improved genetic transformation mediated by Agrobacterium tumefaciens in two rice cultivars. Biotechnology 6:138–147
Allnutt FCT, Kyle DJ, Grossman AR, Apt KE (2000) Methods and tools for transformation of eukaryotic algae. United States of America Patent Number 6027900
Andersen RA, Berges JA, Harrison PJ, Watanabe MM (2005) Recipes for freshwater and seawater media. In: Andersen RA (ed) Algal culturing techniques. Elsevier, Amsterdam, The Netherlands, pp 429–538
Anila N, Chandrashekar A, Ravishankar GA, Sarada R (2011) Establishment of Agrobacterium tumefaciens-mediated genetic transformation in Dunaliella bardawil. Eur J Phycol 46(1):36–44
Anwaruzzaman N, Chin BL, Li XP, Lohr M, Martínez DA, Niyogi KK (2004) Genomic analysis of mutants affecting xanthophylls biosynthesis and regulation of photosynthetic light harvesting in Chlamydomonas reinhardtii. Photosynth Res 82:265–276
Apt KE, Kroth-Pancic PG, Grossman AR (1996) Stable nuclear transformation of the diatom Phaeodactylum tricornutum. Mol Gen Genet 252:572–579
Barik DP, Mohapatra U, Chand PK (2005) Transgenic grasspea (Lathyrus sativus L.): factors influencing Agrobacterium-mediated transformation and regeneration. Plant Cell Rep 24:523–531
Benemann JR (1992) Microalgae aquaculture feeds. J Appl Phycol 4:233–245
Borowitzka MA (1997) Algae for aquaculture: opportunities and constraints. J Appl Phycol 9:393–401
Brown M, Robert R (2002) Preparation and assessment of microalgal concentrates as feeds for larval and juvenile Pacific oyster (Crassostrea gigas). Aquaculture 207:289–309
Cha TS, Chen CF, Yee W, Aziz A, Loh SH (2011) Cinnamic acid, coumarin and vanillin: alternative phenolic compounds for efficient Agrobacterium-mediated transformation of the unicellular green alga, Nannochloropsis sp. J Microbiol Meth 84:430–434
Cha TS, Yee W, Aziz A (2012) Assessment of factors affecting Agrobacterium-mediated genetic transformation of the unicellular green algae, Chlorella vulgaris. World J Microbiol Biotechnol 28:1771–1779
Chamovitz D, Sandmann G, Hirschberg J (1993) Molecular and biochemical characterization of herbicide-resistant mutants of cyanobacteria reveals that phytoene desaturation is a rate-limiting step in carotenoid biosynthesis. J Biol Chem 268:17348–17353
Cheng R, Ma R, Li K, Rong H, Lin X, Wang Z, Yang S, Ma Y (2012) Agrobacterium tumefaciens mediated transformation of marine microalgae Schizochytrium. Microbiol Res 167(3):179–186
Courchesne NMD, Parisien A, Wang B, Lan CQ (2009) Enhancement of lipid production using biochemical, genetic and transcription factor engineering approaches. J Biotechnol 141:31–41
Cunningham FX, Gantt E (1998) Genes and enzymes of carotenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol 49:557–583
Fullner KJ, Nester EW (1996) Temperature affects the T-DNA transfer machinery of Agrobacterium tumefaciens. J Bacteriol 178:1498–1504
Geng D, Wang Y, Wang P, Li W, Sun Y (2003) Stable expression of hepatitis B surface antigen gene in Dunaliella salina (Chlorophyta). J Appl Phycol 15:451–456
Hamilton CM, Frary A, Lewis C, Tanksley S (1996) Stable transfer of intact high molecular weight DNA into plant chromosomes. Proc Natl Acad Sci U S A 93:9975–9979
Hansen G, Shillito RD, Chilton MD (1997) T-strand integration in maize protoplasts after codelivery of a T-DNA substrate and virulence genes. Proc Natl Acad Sci U S A 94:11726–11730
Hawkins RL, Nakamura M (1999) Expression of human growth hormone by the eukaryotic alga, Chlorella. Curr Microbiol 38:335–341
Hiei Y, Ohta S, Komari T, Komashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6:271–282
Hoefgen R, Willmitzer L (1988) Storage of competent cells for Agrobacterium transformation. Nucleic Acid Res 16:9877
Hu Z, Wu Y, Li W, Gao H (2006) Factors affecting Agrobacterium mediated genetic transformation of Lycium barbarum L. In Vitro Cell Dev Biol Plant 42:461–466
Humara JM, López M, Ordás RJ (1999) Agrobacterium tumefaciens mediated transformation of Pinus pinea L. cotyledons: an assessment of factors influencing the efficiency of uidA gene transfer. Plant Cell Rep 19:51–58
Jarvis EE, Brown LM (1991) Transient expression of firefly luciferase in protoplasts of the green alga Chlorella ellipsoidea. Curr Genet 19:317–321
Jin S, Song Y, Deng W, Gordon MP, Nester EW (1993) The regulatory VirA protein of Agrobacterium does not function at elevated temperatures. J Bacteriol 175:6830–6835
Jin ES, Polle JEW, Melis A (2001) Involvement of zeaxanthin and of the Cbr protein in the repair of photosystem II from photoinhibition in the green alga Dunaliella salina. Biochim Biophys Acta 1506:244–259
Kathiresan S, Chandrashekar A, Ravishankar GA, Sarada R (2009) Agrobacterium-mediated transformation of the green alga Haematococcus pluvialis (Chlorophyceae, Volvocales). J Phycol 45:642–649
Khozin-Goldberg I, Iskandarov U, Cohen Z (2011) LC-PUFA from photosynthetic microalgae: occurrence, biosynthesis, and prospects in biotechnology. Appl Microbiol Biotechnol 91:905–915
Kumar SV, Rajam MV (2007) Induction of Agrobacterium tumefaciens vir genes by the green alga, Chlamydomonas reinhardtii. Curr Sci 92(12):1727–1729
Kumar SV, Misquitta RW, Reddy VS, Rao BJ, Rajam MV (2004) Genetic transformation of the green alga-Chlamydomonas reinhardtii by Agrobacterium tumefaciens. Plant Sci 166:731–738
León-Bañares R, González-Ballester D, Galván A, Fernández E (2004) Transgenic microalgae as green cell-factories. Trends Biotechnol 22(1):44–52
Lin JJ, Ma J, Garcia-Assad N, Kuo J (1996) Hygromycin as an efficient antibiotic for the selection of transgenic plants. Focus 18:47–49
Liu J, Zhong Y, Sun Z, Huang J, Sandmann G, Chen F (2010) One amino acid substitution in phytoene desaturase makes Chlorella zofingiensis resistant to norflurazon and enhances the biosynthesis of astaxanthin. Planta 232:61–67
Liu J, Gerken H, Huang J, Chen F (2013) Engineering of an endogenous phytoene desaturase gene as a dominant selectable marker for Chlamydomonas reinhardtii transformation and enhanced biosynthesis of carotenoids. Proc Biochem 48:788–795
Meesapyodsuk D, Qiu X (2012) The front-end desaturease: structure, function, evolution and biotechnical use. Lipids 47:227–237
Pulz O, Gross W (2004) Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol 65:635–648
Rosenberg JN, Oyler GA, Wilkinson L, Betenbaugh MJ (2008) A green light for engineered algae: redirecting metabolism to fuel a biotechnology revolution. Curr Opin Biotechnol 19(5):430–436
Shrawat AK, Becker D, Lörz H (2007) Agrobacterium tumefaciens mediated genetic transformation of barley (Hordeum vulgare L.). Plant Sci 172:281–290
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96
Stachel SE, Messens E, Van Montagu M, Zambryski P (1985) Identification of the signal molecules produced by wounded plant cells that activate T-DNA transfer in Agrobacterium tumefaciens. Nature 318:624–629
Steinbrenner J, Sandmann G (2006) Transformation of the green alga Haematococcus pluvialis with a phytoene desaturase for accelerated astaxanthin biosynthesis. Appl Environ Microbiol 72:7477–7484
Tan CP, Qin S, Zhang Q, Jiang P, Zhao FQ (2005) Establishment of a micro-particle bombardment transformation system for Dunaliella salina. J Microbiol 43:361–365
Tzfira T, Citovsky V (2006) Agrobacterium-mediated genetic transformation of plants: biology and biotechnology. Curr Opin Biotechnol 17:147–154
Walker TL, Purton S, Becker DK, Collet C (2005) Microalgae as bioreactors. Plant Cell Rep 24(11):629–641
Yago T, Arakawa H, Morinaga T, Yoshie-Stark Y, Yoshioka M (2011) Effect of wavelength of intermittent light on the growth and fatty acid profile of the haptophyte Isochrysis galbana. Glob Chang: Mank-Mar Environ Interact 43–45
Acknowledgment
Binod Prasad acknowledges National Institute for International Education (NIIED, South Korea) for a fellowship. The authors are thankful to Dr. J. Steinbrenner (Universität Konstanz, Germany) and Prof. Choi PS (Nambu University, South Korea) for kindly providing the plasmid pPLAT-pds-L504R and Agrobacterium tumefaciens strain LBA 4404, respectively. This work was supported by Busan Metropolitan City, Korea, and Federal Ministry of Education and Research, Germany, which is greatly appreciated by the authors.
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prasad, B., Vadakedath, N., Jeong, HJ. et al. Agrobacterium tumefaciens-mediated genetic transformation of haptophytes (Isochrysis species). Appl Microbiol Biotechnol 98, 8629–8639 (2014). https://doi.org/10.1007/s00253-014-5900-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5900-7