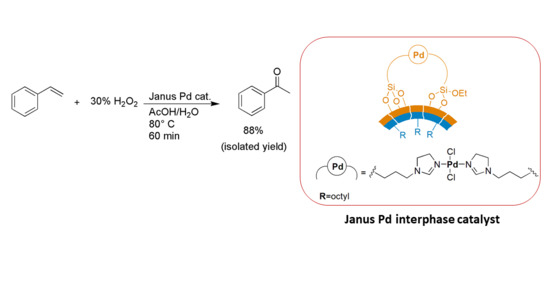
Fast and Selective Aqueous-Phase Oxidation of Styrene to Acetophenone Using a Mesoporous Janus-Type Palladium Catalyst
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. General Methods
4.2. Synthesis of Material 1
4.3. Synthesis of the Palladium Precursor

4.4. Synthesis of Catalyst 2
4.5. Typical Procedure for Palladium-Catalyzed Oxidation of Styrene Using 30% H2O2
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Smidt, J.; Hafner, W.; Jira, R.; Sieber, R.; Sedlmeier, J.; Sabel, A. The Oxidation of Olefins with Palladium Chloride Catalysts. Angew. Chem. Int. Ed. 1962, 1, 80–88. [Google Scholar] [CrossRef]
- Tsuji, J. Synthetic Applications of the Palladium-Catalyzed Oxidation of Olefins to Ketones. Synthesis 1984, 369–384. [Google Scholar] [CrossRef]
- Zhang, Z.; Kumamoto, Y.; Hashiguchi, T.; Mamba, T.; Murayama, H.; Yamamoto, E.; Ishida, T.; Honma, T.; Tokunaga, M. Wacker Oxidation of Terminal Alkenes Over ZrO2-Supported Pd Nanoparticles Under Acid- and Cocatalyst-Free Conditions. ChemSusChem 2017, 10, 3482–3489. [Google Scholar] [CrossRef] [PubMed]
- Donck, S.; Gravel, E.; Shah, N.; Jawale, D.V.; Doris, E.; Namboothiri, I.N.N. Tsuji–Wacker Oxidation of Terminal Olefins using a Palladium–Carbon Nanotube Nanohybrid. ChemCatChem 2015, 7, 2318–2322. [Google Scholar] [CrossRef]
- Speziali, M.G.; Costa, V.V.; Robles-Dutenhefner, P.A.; Gusevskaya, E.V. Aerobic Palladium(II)/Copper(II)-Catalyzed Oxidation of Olefins under Chloride-Free Nonacidic Conditions. Organometallics 2009, 28, 3186–3192. [Google Scholar] [CrossRef]
- Wang, J.; Cai, F.; Wang, E.; He, L. Supercritical carbon dioxide and poly(ethylene glycol): An environmentally benign biphasic solvent system for aerobic oxidation of styrene. Green Chem. 2007, 9, 882–887. [Google Scholar] [CrossRef]
- Roussel, M.; Mimoun, H. Palladium-catalyzed oxidation of terminal olefins to methyl ketones by hydrogen peroxide. J. Org. Chem. 1980, 45, 5387–5390. [Google Scholar] [CrossRef]
- Xie, M.; Yang, X.; Wu, C. A metalloporphyrin functionalized metal–organic framework for selective oxidization of styrene. Chem. Commun. 2011, 47, 5521–5523. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, J.; Peng, X. Efficient Palladium(0) supported on reduced graphene oxide for selective oxidation of olefins using graphene oxide as a ‘solid weak acid’. Catal. Commun. 2019, 122, 73–78. [Google Scholar] [CrossRef]
- Keshipour, S.; Nadervand, S. Fe3O4 nanoparticles as a new efficient co-catalyst for Pd(II) in Wacker oxidation of styrene using H2O2 as an oxidant. RSC Adv. 2015, 5, 47617–47620. [Google Scholar] [CrossRef]
- Liu, W.; Huang, J.; Yang, Q.; Wang, S.; Sun, X.; Zhang, W.; Liu, J.; Huo, F. Multi-shelled Hollow Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2017, 56, 5512–5516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Liu, L.; Han, Z. Palladium nanoparticles supported on UiO-66-NH2 as heterogeneous catalyst for epoxidation of styrene. Inorg. Chem. Commun. 2019, 100, 51–55. [Google Scholar] [CrossRef]
- Cavani, F.; Teles, J.H. Sustainability in Catalytic Oxidation: An Alternative Approach or a Structural Evolution? ChemSusChem 2009, 2, 508–534. [Google Scholar] [CrossRef] [PubMed]
- Mitsudome, T.; Umetani, T.; Nosaka, N.; Mori, K.; Mizugaki, T.; Ebitani, K.; Kaneda, K. Convenient and Efficient Pd-Catalyzed Regioselective Oxyfunctionalization of Terminal Olefins by Using Molecular Oxygen as Sole Reoxidant. Angew. Chem. Int. Ed. 2006, 45, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Venkataramanan, N.S.; Kawanami, H.; Ikushima, Y. Selective oxidation of styrene to acetophenone over supported Au–Pd catalyst with hydrogen peroxide in supercritical carbon dioxide. Green Chem. 2007, 9, 1352–1355. [Google Scholar] [CrossRef]
- Xia, X.; Gao, X.; Xu, J.; Hu, C.; Peng, X. Selective Oxidation of Styrene Derivatives to Ketones over Palladium(0)/Carbon with Hydrogen Peroxide as the Sole Oxidant. Synlett 2017, 28, 607–610. [Google Scholar]
- Byun, S.; Chung, J.; Jang, Y.; Kwon, J.; Hyeon, T.; Kim, B.M. Highly selective Wacker oxidation of terminal olefins using magnetically recyclable Pd–Fe3O4 heterodimer nanocrystals. RSC Adv. 2013, 3, 16296–16299. [Google Scholar] [CrossRef]
- Cao, Q.; Bailie, D.S.; Fu, R.; Muldoon, M.J. Cationic palladium(II) complexes as catalysts for the oxidation of terminal olefins to methyl ketones using hydrogen peroxide. Green Chem. 2015, 17, 2750–2757. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Geng, W.; Zhou, J.; Luo, W.; Wang, F.; Wang, L.; Tsang, S.C. Synthesis of multicarboxylic acid appended imidazolium ionic liquids and their application in palladium-catalyzed selective oxidation of styrene. New J. Chem. 2007, 31, 2088–2094. [Google Scholar] [CrossRef]
- Walker, K.L.; Dornan, L.M.; Zare, R.N.; Waymouth, R.M.; Muldoon, M.J. Mechanism of Catalytic Oxidation of Styrenes with Hydrogen Peroxide in the Presence of Cationic Palladium(II) Complexes. J. Am. Chem. Soc. 2017, 139, 12495–12503. [Google Scholar] [CrossRef] [Green Version]
- Feng, B.; Hou, Z.; Wang, X.; Hu, Y.; Li, H.; Qiao, Y. Selective aerobic oxidation of styrene to benzaldehyde catalyzed by water-soluble palladium(II) complex in water. Green Chem. 2009, 11, 1446–1452. [Google Scholar] [CrossRef]
- Lindner, E.; Schneller, T.; Auer, F.; Mayer, H.A. Chemistry in Interphases—A New Approach to Organometallic Syntheses and Catalysis. Angew. Chem. Int. Ed. 1999, 38, 2154–2174. [Google Scholar] [CrossRef]
- Wu, Z.; Li, L.; Liao, T.; Chen, X.; Jiang, W.; Luo, W.; Yang, J.; Sun, Z. Janus nanoarchitectures: From structural design to catalytic applications. Nano Today 2018, 22, 62–82. [Google Scholar] [CrossRef]
- Vafaeezadeh, M.; Thiel, W.R. Janus interphase catalysts for interfacial organic reactions. J. Mol. Liquid 2020, 315, 113735. [Google Scholar] [CrossRef]
- Ji, X.; Zhang, Q.; Qu, X.; Wang, Q.; Song, X.; Liang, F.; Yang, Z. Poly(ionic liquid) Janus nanosheets towards dye degradation. RSC Adv. 2015, 5, 21877–21880. [Google Scholar] [CrossRef]
- Kong, X.; Wu, C.; Feng, L.; Qu, J.; Liu, P.; Wang, X.; Zhang, X. Silica-based hierarchical porous Janus microcapsules: Construction and support of Au nano-particle catalyst inside. Chem. Commun. 2017, 53, 8054–8057. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Tang, Z.; Fu, W.; Wang, W.; Tan, R.; Yin, D. An ionic liquid-functionalized amphiphilic Janus material as a Pickering interfacial catalyst for asymmetric sulfoxidation in water. Chem. Commun. 2019, 55, 592–595. [Google Scholar] [CrossRef]
- Yuan, K.; Li, Y.; Huang, X.; Liang, Y.; Liu, Q.; Jiang, G. Templated synthesis of a bifunctional Janus graphene for enhanced enrichment of both organic and inorganic targets. Chem. Commun. 2019, 55, 4957–4960. [Google Scholar] [CrossRef] [PubMed]
- Vafaeezadeh, M.; Breuninger, P.; Lösch, P.; Wilhelm, C.; Ernst, S.; Antonyuk, S.; Thiel, W.R. Janus Interphase Organic-Inorganic Hybrid Materials: Novel Water-Friendly Heterogeneous Catalysts. ChemCatChem 2019, 11, 2304–2312. [Google Scholar] [CrossRef]
- Vafaeezadeh, M.; Wilhelm, C.; Breuninger, P.; Ernst, S.; Antonyuk, S.; Thiel, W.R. A Janus-type Heterogeneous Surfactant for Adipic Acid Synthesis. ChemCatChem 2020, 12, 2695–2701. [Google Scholar] [CrossRef]
- Vafaeezadeh, M.; Schaumlöffel, J.; Lösch, A.; De Cuyper, A.; Thiel, W.R. Dinuclear Copper Complex Immobilized on a Janus-Type Material as an Interfacial Heterogeneous Catalyst for Green Synthesis. ACS Appl. Mater. Interfaces 2021, 13, 33091–33101. [Google Scholar] [CrossRef]
- Karimi, B.; Khorasani, M. Selectivity Adjustment of SBA-15 Based Tungstate Catalyst in Oxidation of Sulfides by Incorporating a Hydrophobic Organic Group inside the Mesochannels. ACS Catal. 2013, 3, 1657–1664. [Google Scholar] [CrossRef]
- Tsuji, J.; Nagashima, H.; Hori, K. A new preparative method for 1,3-dicarbonyl compounds by the regioselective oxidation of α,β-unsaturated carbonyl compounds, catalyzed by PdCl2 using hydroperoxides as the reoxidant of Pd0. Chem. Lett. 1980, 9, 257–260. [Google Scholar] [CrossRef]






| Entry | Catalyst (amount) | Oxidant (equiv.) | Time (h) | Solvent | Temp. (°C) | Ap (%) | Ba (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | – | 30% H2O2 (3) | 1 | AcOH/H2O a | 80 | – | 12 b | this work |
| 2 | PdCl2 (1 mol%) | 30% H2O2 (3) | 1 | AcOH/H2O | 80 | 67 b | 8 b | this work |
| 3 | 2 (1 mol%) | 30% H2O2 (3) | 0.5 | AcOH/H2O | 80 | 76 b | 9 b | this work |
| 4 | 2 (1 mol%) | 30% H2O2 (3) | 1 | AcOH/H2O | 80 | 88 b | 9 b | this work |
| 5 | 2 (1 mol%) | 30% H2O2 (3) | 1 | AcOH | 80 | 82 b | 15 b | this work |
| 6 | 2 (1 mol%) + CuCl (10 mol%) | O2 (balloon) | 16 | AcOH/H2O | 80 | 63 b | 30 b | this work |
| 7 | 2 (1 mol%) | TBHP c (2) | 16 | CH3CN | 80 | – | 37 b,d | this work |
| 8 | 2 (1 mol%) | 35% UHP (3) | 16 | AcOH/H2O | 80 | 47 b | 19 b | this work |
| 9 | Na2PdCl4 (10 mol%) | 30% H2O2 (n.d.) | 2.5 | aq. NMP | r.t. | 55 | n.d. | [33] |
| 10 | Pd0/C (5 mol%) | 30% H2O2 (6) | 8 | CH3CN | 65 | 90 e | n.d. | [16] |
| 11 | Pd/Al2O3 (2.5 wt%) | 30% H2O2 (4) | 3 | scCO2 | 120 | 83 f | 6 | [15] |
| 12 | (PBO)Pd(CH3CN)2(OTf)2 (1 mol%) | 50% H2O2 (5) | 24 | CH3CN | r.t. | 80 g | n.d. | [18] |
| 13 | Pd0/RGO(0.01 g), GO (0.01 g) | 30% H2O2 (10) | 12 | CH3CN/H2O | 55 | 93 | n.d. | [9] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vafaeezadeh, M.; Saynisch, R.; Lösch, A.; Kleist, W.; Thiel, W.R. Fast and Selective Aqueous-Phase Oxidation of Styrene to Acetophenone Using a Mesoporous Janus-Type Palladium Catalyst. Molecules 2021, 26, 6450. https://doi.org/10.3390/molecules26216450
Vafaeezadeh M, Saynisch R, Lösch A, Kleist W, Thiel WR. Fast and Selective Aqueous-Phase Oxidation of Styrene to Acetophenone Using a Mesoporous Janus-Type Palladium Catalyst. Molecules. 2021; 26(21):6450. https://doi.org/10.3390/molecules26216450
Chicago/Turabian StyleVafaeezadeh, Majid, Ranja Saynisch, Andrea Lösch, Wolfgang Kleist, and Werner R. Thiel. 2021. "Fast and Selective Aqueous-Phase Oxidation of Styrene to Acetophenone Using a Mesoporous Janus-Type Palladium Catalyst" Molecules 26, no. 21: 6450. https://doi.org/10.3390/molecules26216450