Anti-Inflammatory Effects of C1q/Tumor Necrosis Factor-Related Protein 3 (CTRP3) in Endothelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Recombinant CTRP3
2.2. Mice and Cells
2.2.1. LPS-Induced Systemic Inflammatory Response Syndrome (SIRS) Model
2.2.2. Murine Endothelial Cells
2.3. Isolation of Tissues and Organs
2.4. Real-Time PCR
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Cell Adhesion Assay
2.7. Statistical Analysis
3. Results
3.1. Ctrp3 Is Expressed in a Wide Variety of Tissues and Organs and in Endothelial Cells
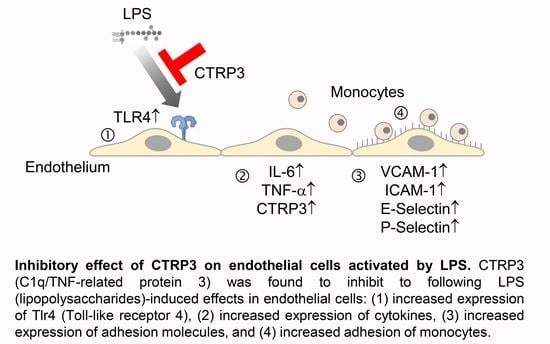
3.2. LPS-Induced Cytokine Expression in Endothelial Cells Is Inhibited by CTRP3
3.3. LPS-Induced Endothelial Adhesion Molecule Expression and Adhesion of Monocytic Cells Is Inhibited by CTRP3
3.4. Effects of CTRP3 In Vivo and on Tlr4 Expression in Endothelial Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A



References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Santilli, F.; D’Ardes, D.; Guagnano, M.T.; Davi, G.; Santilli, D.D.F. Metabolic Syndrome: Sex-Related Cardiovascular Risk and Therapeutic Approach. Curr. Med. Chem. 2017, 24, 2602–2627. [Google Scholar] [CrossRef]
- Engin, A. The Definition and Prevalence of Obesity and Metabolic Syndrome. In Obesity and Lipotoxicity; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2017; Volume 960, pp. 1–17. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Engin, A. Endothelial dysfunction in obesity. In Obesity and Lipotoxicity; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2017; Volume 960, pp. 345–379. [Google Scholar]
- Luo, L.; Liu, M. Adipose tissue in control of metabolism. J. Endocrinol. 2016, 231, R77–R99. [Google Scholar] [CrossRef] [Green Version]
- Kahn, C.R.; Wang, G.; Lee, K.Y. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J. Clin. Investig. 2019, 129, 3990–4000. [Google Scholar] [CrossRef] [PubMed]
- Schäffler, A.; Schölmerich, J. Innate immunity and adipose tissue biology. Trends Immunol. 2010, 31, 228–235. [Google Scholar] [CrossRef]
- Reilly, S.; Saltiel, A.R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 2017, 13, 633–643. [Google Scholar] [CrossRef]
- Funcke, J.-B.; Scherer, P.E. Beyond adiponectin and leptin: Adipose tissue-derived mediators of inter-organ communication. J. Lipid Res. 2019, 60, 1648–1697. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.; Wei, Z.; Wong, G.W. C1q/TNF-related Protein-3 (CTRP3), a Novel Adipokine That Regulates Hepatic Glucose Output. J. Biol. Chem. 2010, 285, 39691–39701. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Wang, F.; Chu, Z.; Shi, X.; Sun, L.; Lv, H.; Zhou, W.; Shen, J.; Chen, L.; Hou, M. Serum CTRP3 Levels in Obese Children: A Potential Protective Adipokine Of Obesity, Insulin Sensitivity And Pancreatic β Cell Function. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 1923–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, T.; Wakisaka, S. Deficiency of C1q/TNF-related protein 3 (CTRP3) decreases adipose tissue weight in diet-induced obesity mice. Mol. Biol. Rep. 2020, 47, 9219–9224. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Roderfeld, M.; Gehl, J.; Roeb, E.; Nist, A.; Chung, H.-R.; Stiewe, T.; Karrasch, T.; Schäffler, A. C1q/TNF-Related Protein 3 (CTRP-3) Deficiency of Adipocytes Affects White Adipose Tissue Mass but Not Systemic CTRP-3 Concentrations. Int. J. Mol. Sci. 2021, 22, 1670. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Li, C.; Zhang, Y.; Zhao, J.; Yang, M.; Tian, M.; Li, L.; Zheng, Y.; Chen, B.; Yang, G. Serum C1q/TNF-related protein-3 (CTRP3) levels are decreased in obesity and hypertension and are negatively correlated with parameters of insulin resistance. Diabetol. Metab. Syndr. 2015, 7, 33. [Google Scholar] [CrossRef] [Green Version]
- Peterson, J.; Seldin, M.M.; Wei, Z.; Aja, S.; Wong, G.W. CTRP3 attenuates diet-induced hepatic steatosis by regulating triglyceride metabolism. Am. J. Physiol. Liver Physiol. 2013, 305, G214–G224. [Google Scholar] [CrossRef] [Green Version]
- Guo, B.; Zhuang, T.; Xu, F.; Lin, X.; Li, F.; Shan, S.-K.; Wu, F.; Zhong, J.-Y.; Wang, Y.; Zheng, M.-H.; et al. New Insights into Implications of CTRP3 in Obesity, Metabolic Dysfunction, and Cardiovascular Diseases: Potential of Therapeutic Interventions. Front. Physiol. 2020, 11, 570270. [Google Scholar] [CrossRef]
- Murayama, M.A.; Kakuta, S.; Maruhashi, T.; Shimizu, K.; Seno, A.; Kubo, S.; Sato, N.; Saijo, S.; Hattori, M.; Iwakura, Y. CTRP3 plays an important role in the development of collagen-induced arthritis in mice. Biochem. Biophys. Res. Commun. 2014, 443, 42–48. [Google Scholar] [CrossRef] [Green Version]
- Petersen, P.S.; Wolf, R.M.; Lei, X.; Peterson, J.; Wong, G.W. Immunomodulatory roles of CTRP3 in endotoxemia and metabolic stress. Physiol. Rep. 2016, 4, e12735. [Google Scholar] [CrossRef]
- Kopp, A.; Bala, M.; Buechler, C.; Falk, W.; Gross, P.; Neumeier, M.; Schölmerich, J.; Schäffler, A. C1q/TNF-Related Protein-3 Represents a Novel and Endogenous Lipopolysaccharide Antagonist of the Adipose Tissue. Endocrinology 2010, 151, 5267–5278. [Google Scholar] [CrossRef]
- Schmid, A.; Kopp, A.; Hanses, F.; Karrasch, T.; Schäffler, A. C1q/TNF-related protein-3 (CTRP-3) attenuates lipopolysaccharide (LPS)-induced systemic inflammation and adipose tissue Erk-1/-2 phosphorylation in mice in vivo. Biochem. Biophys. Res. Commun. 2014, 452, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Compton, S.A.; Cheatham, B. CTRP-3: Blocking a Toll Booth to Obesity-Related Inflammation. Endocrinology 2010, 151, 5095–5097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopp, A.; Bala, M.; Weigert, J.; Büchler, C.; Neumeier, M.; Aslanidis, C.; Schölmerich, J.; Schäffler, A. Effects of the new adiponectin paralogous protein CTRP-3 and of LPS on cytokine release from monocytes of patients with type 2 diabetes mellitus. Cytokine 2010, 49, 51–57. [Google Scholar] [CrossRef]
- Shao, Y.; Saredy, J.; Yang, W.Y.; Sun, Y.; Lu, Y.; Saaoud, F.; Iv, C.D.; Johnson, C.; Xu, K.; Jiang, X.; et al. Vascular Endothelial Cells and Innate Immunity. Arter. Thromb. Vasc. Biol. 2020, 40, e138–e152. [Google Scholar] [CrossRef]
- Weigert, J.; Neumeier, M.; Schäffler, A.; Fleck, M.; Schölmerich, J.; Schütz, C.; Buechler, C. The adiponectin paralog CORS-26 has anti-inflammatory properties and is produced by human monocytic cells. FEBS Lett. 2005, 579, 5565–5570. [Google Scholar] [CrossRef] [Green Version]
- Vlacil, A.-K.; Vollmeister, E.; Bertrams, W.; Schoesser, F.; Oberoi, R.; Schuett, J.; Schuett, H.; Huehn, S.; Bedenbender, K.; Schmeck, B.; et al. Identification of microRNAs involved in NOD-dependent induction of pro-inflammatory genes in pulmonary endothelial cells. PLoS ONE 2020, 15, e0228764. [Google Scholar] [CrossRef] [PubMed]
- Troidl, K.; Schubert, C.; Vlacil, A.-K.; Chennupati, R.; Koch, S.; Schütt, J.; Oberoi, R.; Schaper, W.; Schmitz-Rixen, T.; Schieffer, B.; et al. The Lipopeptide MALP-2 Promotes Collateral Growth. Cells 2020, 9, 997. [Google Scholar] [CrossRef]
- Williams, M.R.; Azcutia, V.; Newton, G.; Alcaide, P.; Luscinskas, F.W. Emerging mechanisms of neutrophil recruitment across endothelium. Trends Immunol. 2011, 32, 461–469. [Google Scholar] [CrossRef] [Green Version]
- Oberoi, R.; Schuett, J.; Schuett, H.; Koch, A.-K.; Luchtefeld, M.; Grote, K.; Schieffer, B. Targeting Tumor Necrosis Factor-α with Adalimumab: Effects on Endothelial Activation and Monocyte Adhesion. PLoS ONE 2016, 11, e0160145. [Google Scholar] [CrossRef]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory Mechanisms Contributing to Endothelial Dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef]
- Park, B.S.; Lee, J.-O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp. Mol. Med. 2013, 45, e66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemichez, E.; Lecuit, M.; Nassif, X.; Bourdoulous, S. Breaking the wall: Targeting of the endothelium by pathogenic bacteria. Nat. Rev. Genet. 2009, 8, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Goulopoulou, S.; McCarthy, C.G.; Webb, R.C. Toll-like Receptors in the Vascular System: Sensing the Dangers Within. Pharmacol. Rev. 2015, 68, 142–167. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmid, A.; Vlacil, A.-K.; Schuett, J.; Karrasch, T.; Schieffer, B.; Schäffler, A.; Grote, K. Anti-Inflammatory Effects of C1q/Tumor Necrosis Factor-Related Protein 3 (CTRP3) in Endothelial Cells. Cells 2021, 10, 2146. https://doi.org/10.3390/cells10082146
Schmid A, Vlacil A-K, Schuett J, Karrasch T, Schieffer B, Schäffler A, Grote K. Anti-Inflammatory Effects of C1q/Tumor Necrosis Factor-Related Protein 3 (CTRP3) in Endothelial Cells. Cells. 2021; 10(8):2146. https://doi.org/10.3390/cells10082146
Chicago/Turabian StyleSchmid, Andreas, Ann-Kathrin Vlacil, Jutta Schuett, Thomas Karrasch, Bernhard Schieffer, Andreas Schäffler, and Karsten Grote. 2021. "Anti-Inflammatory Effects of C1q/Tumor Necrosis Factor-Related Protein 3 (CTRP3) in Endothelial Cells" Cells 10, no. 8: 2146. https://doi.org/10.3390/cells10082146