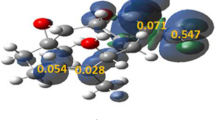
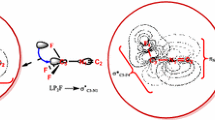
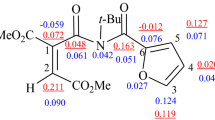
Abstract
Isocyanide-nitrile rearrangement has long been a continuing and interesting topic. A series of nitriles and isocyanides with the substituents of R = -AlH2, -BeH, -BH2, -C≡CH, -CF3, -CH3, -Cl, -C≡N, -COOH, -F, -H, Li, -MgH, -Na, -NH2, -NO2, -OH, -PH2, -SH, -SiH3, and -CH = CH2 were investigated systematically based on full optimization at B3LYP-D3(BJ)/def2-QZVP level, and the isomerization energies from R–C≡N to:C = N-R were estimated. The substituent effect and bonding characters were analyzed by surface ESP colored van der Waals surfaces in conjunction with the global and local electrostatic extrema.
Graphical abstract



Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Huang K, Couchman SA, Wilson DJD, Dutton JL, Martin CD (2015) Reactions of imines, nitriles, and isocyanides with pentaphenylborole: Coordination, ring expansion, C-H bond activation, and hydrogen migration reactions. Inorg Chem 54:8957–8968
Kosar N, Ayub K, Gilani MA, Mahmood T (2019) Benchmark DFT studies on C-CN homolytic cleavage and screening the substitution effect on bond dissociation energy. J Mol Model 25:47
Sarkar N, Bera S, Nembenna S (2020) Aluminum-catalyzed selective hydroboration of nitriles and alkynes: A multifunctional catalyst. J Org Chem 85:4999–5009
Li Y, Wang X, Wang H, Ni Y, Wang H (2021) Influence of halogen atom substitution and neutral HCN/anion CN− Lewis base on the triel-bonding interactions. J Mol Model 27:93
Rüchardt C, Meier M, Haaf K, Pakusch J, Wolber EKA, Müller B (1991) The isocyanide–cyanide rearrangement; Mechanism and preparative applications. Angew Chem Int Ed Engl 30:893–901
Sung K (1999) Substituent effects on stability and isomerization energies of isocyanides and nitriles. J Org Chem 64:8984–8989
Nguyen PT, Palmer WS, Woerpel KA (1999) Stereospecific and regioselective isocyanide insertions into siliranes and reactions of the resulting iminosilacyclobutanes. J Org Chem 64:1843–1848
Huang R, Shen Q, Zhang C, Zhang S, Xu H (2020) Studies on the mechanism of the transition metal-catalyzed reaction of organonitrile with sodium azide. Acta Chim Sinica 78:565–571 ((In Chinese))
Gutiérrez-Oliva S, Díaz S, Toro-Labbé A, Lane P, Murray JS, Politzer P (2014) Revisiting the seemingly straightforward hydrogen cyanide/hydrogen isocyanide isomerisation. Mol Phys 112:349–354
Pracna P, Urban J, Votava O, Meltzerová Z, Urban Š, Horneman V-M (2011) Rotational and rovibrational spectroscopy of the v8 = 1 and 2 vibrational states of CH3NC. Mol Phys 109:2237–2243
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2013) Gaussian 09, Revision D.01. Gaussian Inc., Wallingford
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr G (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Grimme S, Ehrlich S, Goerigk L (2011) Effect of the damping function in dispersion corrected density functional theory. J Comp Chem 32:1456–1465
Lu T, Chen F (2013) Revealing the nature of intermolecular interaction and configurational preference of the nonpolar molecular dimers (H2)2, (N2)2, and (H2)(N2). J Mol Model 19:5387–5395
Witte J, Goldey M, Neaton JB, Head-Gordon M (2015) Beyond energies: Geometries of nonbonded molecular complexes as metrics for assessing electronic structure approaches. J Chem Theory Comput 11:1481–1492
Di Meo F, Bayach I, Trouillas P, Sancho-García J-C (2015) Unraveling the performance of dispersion-corrected functionals for the accurate description of weakly bound natural polyphenols. J Mol Model 21:291
Řezáč J (2020) Non-Covalent Interactions Atlas benchmark data sets 2: Hydrogen bonding in an extended chemical space. J Chem Theory Comput 16:6305–6316
Cheng X (2020) Computational insights into the coupling mechanism of benzoic acid, phenyl alkynyl ether and dihydroisoquinoline catalyzed by silver ion as polarizer and stabilizer. Appl Organometal Chem 34:e5903
Weigend F, Ahlrichs R (2005) Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys Chem Chem Phys 7:3297–3305
Lu T, Chen F (2012) Quantitative analysis of molecular surface based on improved marching tetrahedra algorithm. J Mol Graph Model 38:314–323
Manzetti S, Lu T (2013) The geometry and electronic structure of aristolochic acid: Possible implications for a frozen resonance. J Phys Org Chem 26:473–483
Zhang Z, Lu T, Ding L, Wang G, Wang Z, Zheng B, Liu Y, Ding XL (2020) Cooperativity effects between regium-bonding and pnicogen-bonding interactions in ternary MF···PH3O···MF (M = Cu, Ag, Au): An ab initio study. Mol Phys 118:e1784478
Ren F, Shi W, Cao D, Li Y, Zhang D, Wang X, Shi Z (2020) External electric field reduces the explosive sensitivity: A theoretical investigation into the hydrogen transference kinetics of the NH2NO2∙∙∙H2O complex. J Mol Model 26:351
Zhang Y, Gou R, Chen Y (2021) Theoretical insight on effect of DMSO-acetonitrile co-solvent on the formation of CL-20/HMX cocrystal explosive. J Mol Model 27:8
Lu T, Chen F (2012) Multiwfn: A Multifunctional wave function analyzer. J Comput Chem 33:580–592
Humphrey W, Dalke A, Schulten K (1996) VMD: Visual molecular dynamics. J Mol Graphics 14:33–38
Zhang J (2018) LIBRETA: Computerized optimization and code synthesis for electron repulsion integral evaluation. J Chem Theory Comput 14:572–587
Ehrenson S, Brownlee RTC, Taft RW (1973) A generalized treatment of substituent effects in the benzene series. A statistical analysis by the dual substituent parameter equation (1). Prog Phys Org Chem 10:1–80
Funding
This work was supported by the Natural Science Foundation of Shandong Province (ZR2017LB010) and the Science and Technology Planning Project (Guidance Plan) of Tai’an City (No. 2018GX0041).
Author information
Authors and Affiliations
Contributions
Yanyun Zhao: Software; Formal analysis; Investigation; Writing, original draft. Xueli Cheng: Conceptualization; Methodology; Software; Writing, original draft and editing; Management and responsibility for the research activity planning and execution.
Corresponding author
Ethics declarations
Consent for publication
Written informed consent for publication was obtained from all participants.
Conflicts of interest
The authors declare have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, Y., Cheng, X. Isomerization energies and surface electrostatic potential analyses on nitriles and isocyanides. J Mol Model 27, 257 (2021). https://doi.org/10.1007/s00894-021-04870-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-021-04870-6