Abstract
A reusable, low–cost, and convenient ethylenediamine (EDA)–bound magnetite nanoparticles (MNPs)–based colorimetric sensor has been developed for dual function colorimetric determination of nitroaromatic explosives such as TNT and tetryl. Colorimetric detection of analytes may occur through two independent routes: (1) nano−Fe3O4− EDA− NH2 as σ−donor may interact with the σ− and π−acceptor aromatic−poly(NO2) groups to produce a colored charge−transfer (CT) complex; (2) nano−Fe3O4−EDA−NH2 as a Fenton-type nanozyme may generate reactive species that comprise hydroxyl radicals (•OH) with H2O2 to oxidize 3,3′,5,5′–tetramethylbenzidine (TMB) to a blue-colored diimine (oxTMB−TMB) CT complex, where this color is bleached with TNT/tetryl because of donor−acceptor interactions between the explosive –NO2 groups and the –NH2 group of Fe3O4−EDA nanoparticles of restricted nanozyme activity. Both methods can quantify TNT well below the EPA recommended TNT residential screening level in soil, LOD being in the micromolar range. As EDA was covalently bound to MNPs, the same sensor can be separately reused six times for TNT and eight times for tetryl determination, using method (1). Common metal ions, anions, energetic materials, several camouflage materials, and soil components such as humates did not interfere with the nanosensor performance for TNT and tetryl. The combination of charge−transfer and nanozyme ability of Fe3O4− EDA−NH2 nanoparticles may bring a new approach to dual function colorimetric sensor design. To the best of our knowledge, this is the first dual function colorimetric sensor for TNT and tetryl using the same nanoparticles as sensing elements in two different detection systems involving either formation or bleaching of colored species.
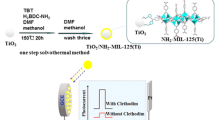
Graphical abstract

The proposed colorimetric sensor can determine nitroaromatic explosives in two different ways: method−1 for TNT and tetryl sensing with EDA–MNPs relies on the donor–acceptor interaction between the electron–deficient nitroaromatics and electron–rich amine groups covalently functionalized on MNPs to produce an absorbance at 512 nm. In method−2, EDA–MNPs having nanozyme activity react with H2O2 to form reactive species that can oxidize TMB to its blue–colored charge–transfer (CT) complex, where TNT and tetryl addition may partially inhibit the nanozyme activity of EDA–MNPs and cause color bleaching (decrement of 650 nm absorbance) by disrupting the CT complex formed from TMB. This is the first dual function colorimetric sensor for nitro explosives uniquely combining charge-transfer and nanozyme ability of EDA–Fe3O4 nanoparticles in the same nano-sensor.







Similar content being viewed by others
Change history
15 September 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00604-021-04955-2
References
Fuller ME, Hatzinger PB, Rungmakol D, Schuster RL, Steffan RJ (2004) Enhancing the attenuation of explosives in surface soils at military facilities: combined sorption and biodegradation. Environ Toxicol Chem 23:313–324. https://doi.org/10.1897/03-187
Lewis TA, Newcombe DA, Crawford RL (2004) Bioremediation of soils contaminated with explosives. J Environ Manag 70:291–307. https://doi.org/10.1016/j.jenvman.2003.12.005
Adams VH (2015) Wildlife toxicity Assesment for N-methyl-N-2,4,6-Tetranitroanaline (tetryl). In: Williams MA, Reddy G, Quinn MJ Jr, Johnson MS (eds) Wildlife toxicity assessments for chemicals of military concern, first edn. Elsevier, UK, Oxford, pp 205–216
Boopathy R (2005) Bioremediation of tetryl-contaminated soil using sequencing batch soil slurry reactor. Int Biodeterior Biodegradation 55:293–297. https://doi.org/10.1016/j.ibiod.2005.03.006
Akhgari F, Fattahi H, Oskei YM (2015) Recent advances in nanomaterial-based sensors for detection of trace nitroaromatic explosives. Sensors Actuators B Chem 221:867–878. https://doi.org/10.1016/j.snb.2015.06.146
Özcan Ç, Üzer A, Durmazel S, Apak R (2019) Colorimetric sensing of nitroaromatic energetic materials using surfactant-stabilized and dithiocarbamate-functionalized gold nanoparticles analytical letters 52:2794–2808. https://doi.org/10.1080/00032719.2019.1608555
Erçağ E, Üzer A, Apak A (2009) Selective spectrophotometric determination of TNT using a dicyclohexylamine-based colorimetric sensor. Talanta 78:772–780. https://doi.org/10.1016/j.talanta.2008.12.042
Puc U, Abina A, Rutar M, Zidanšek A, Jeglič A, Valušis G (2015) Terahertz spectroscopic identification of explosive and drug simulants concealed by various hiding techniques. Appl Opt 54:4495–4502. https://doi.org/10.1364/AO.54.004495
Kendziora CA, Furstenberg R, Papantonakis M, Nguyen V, McGill RA (2016) Broadband infrared imaging spectroscopy for standoff detection of trace explosives. International Society for Optics and Photonics 98362G:9836. https://doi.org/10.1117/12.2224049
Buryakov IA (2011) Detection of explosives by ion mobility spectrometry. J Anal Chem 66:674–694. https://doi.org/10.1134/S1061934811080077
Ewing AV, Kazarian SG (2017) Infrared spectroscopy and spectroscopic imaging in forensic science. Analyst 142:257–272. https://doi.org/10.1039/C6AN02244H
Lopez–Lopez M, Garcia–Ruiz C (2014) Infrared and Raman spectroscopy techniques applied to identification of explosives. TrAC Trends Anal Chem 54:36–44. https://doi.org/10.1016/j.trac.2013.10.011
Sağlam S, Üzer A, Erçağ E, Apak A (2018) Electrochemical determination of TNT, DNT, RDX, and HMX with gold nanoparticles/poly(carbazole-aniline) film–modified glassy carbon sensor electrodes imprinted for molecular recognition of nitroaromatics and nitramines. Anal Chem 90:7364–7370. https://doi.org/10.1021/acs.analchem.8b00715
Sağlam Ş, Üzer A, Tekdemir Y, Erçağ E, Apak R (2018) Electrochemical sensor for nitroaromatic type energetic materials using gold nanoparticles/poly(o-phenylenediamine–aniline) film modified glassy carbon electrode. Talanta 139:181–188. https://doi.org/10.1016/j.talanta.2015.02.059
Kudr J, Haddad Y, Richtera L, Heger Z, Cernak M, Adam V, Zitka O (2017) Magnetic nanoparticles: from design and synthesis to real world applications. Nanomaterials 7:243. https://doi.org/10.3390/nano7090243
Beveridge JS, Stephens JR, Williams ME (2011) The use of magnetic nanoparticles in analytical chemistry. Annu Rev Anal Chem 4:251–273. https://doi.org/10.1146/annurev-anchem-061010-114041
Su L, Feng J, Zhou X, Ren C, Li H, Chen X (2012) Colorimetric detection of urine glucose based ZnFe2O4 magnetic nanoparticles. Anal Chem 84:5753–5758. https://doi.org/10.1021/ac300939z
Zhang H, Harpster MH, Park HJ, Johnson PA, Wilson WC (2010) Surface-enhanced Raman scattering detection of DNA derived from the West Nile virus genome using magnetic capture of Raman-active gold nanoparticles. Anal Chem 83:254–260. https://doi.org/10.1021/ac1023843
Shen YF, Tang J, Nie ZH, Wang YD, Ren Y, Zuo L (2009) Preparation and application of magnetic Fe3O4 nanoparticles for wastewater purification. Sep Purif Technol 68:312–319. https://doi.org/10.1016/j.seppur.2009.05.020
Faraji M, Yamini Y, Rezaee M (2010) Magnetic nanoparticles: synthesis, stabilization, functionalization, characterization, and applications. J Iran Chem Soc 7:1–37. https://doi.org/10.1007/BF03245856
Josephy PD, Eling T, Mason RP (1982) The horseradish peroxidase-catalyzed oxidation of 3,5,3′,5′-tetramethylbenzidine. Free radical and charge-transfer complex intermediates. J Biol Chem 257:3669–3675. https://doi.org/10.1016/S0021-9258(18)34832-4
Frey A, Meckelein B, Externest D, Schmidt MA (2000) A stable and highly sensitive 3,3′,5,5′-tetramethylbenzidine-based substrate reagent for enzyme-linked immunosorbent assays. J Immunol Methods 233:47–56. https://doi.org/10.1016/S0022-1759(99)00166-0
Gao L, Zhuang J, Nie L, Zhang J, Zhang Y, Gu N, Wang T, Feng J, Yang D, Perrett S, Yan X (2007) Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol 2:577–583. https://doi.org/10.1038/nnano.2007.260
Can Z, Üzer A, Türkekul K, Erçağ E, Apak R (2015) Determination of triacetone triperoxide with a N,N-dimethyl-p-phenylenediamine sensor on Nafion using Fe3O4 magnetic nanoparticles. Anal Chem 87:9589–9594. https://doi.org/10.1021/acs.analchem.5b01775
Choodum A, Kanatharana P, Wongniramaikul W, Nic Daeid N (2013) Using the iPhone as a device for a rapid quantitative analysis of trinitrotoluene in soil. Talanta 115:143–149. https://doi.org/10.1016/j.talan”ta.2013.04.037
Sharma SP, Lahiri SC (2008) Absorption spectroscopic and FTIR studies on EDA complexes between TNT (2,4,6-trinitrotoluene) with amines in DMSO and determination of the vertical electron affinity of TNT. Spectrochim Acta MolBiomol 70:144–153. https://doi.org/10.1016/j.saa.2007.07.025
Xie C, Zhang Z, Wang D, Guan G, Gao D, Liu J (2006) Surface molecular self-assembly strategy for TNT imprinting of polymer nanowire/nanotube arrays. Anal Chem 78:8339–8346. https://doi.org/10.1021/ac0615044
Riskin M, Tel-Vered R, Bourenko T, Granot E, Willner I (2008) Imprinting of molecular recognition sites through electropolymerization of functionalized Au nanoparticles: development of an electrochemical TNT sensor based on π-donor−acceptor interactions. J Am Chem Soc 130:9726–9733. https://doi.org/10.1021/ja711278c
Jiang Y, Zhao H, Zhu N, Lin Y, Yu P, Mao L (2008) A simple assay for direct colorimetric visualization of trinitrotoluene at Picomolar levels using gold nanoparticles. Angew Chem Int Ed 47:8601–8604. https://doi.org/10.1002/ange.200804066
Tu R, Liu B, Wang Z, Gao D, Wang F, Fang Q, Zhang Z (2008) Amine-capped ZnS−Mn2+ nanocrystals for fluorescence detection of trace TNT explosive. Anal Chem 80:3458–3465. https://doi.org/10.1021/ac800060f
Qi W, Xu M, Pang L, Liu Z, Zhang W, Majeed S, Xu G (2014) Electrochemiluminescence detection of TNT by resonance energy transfer through the formation of a TNT–amine complex. Chem–Eur J 20:4829–4835. https://doi.org/10.1002/chem.201303710
Songvorawit N, Tuitemwong K, Tuitemwong P (2011) Single step synthesis of amino-functionalized magnetic nanoparticles with polyol technique at low temperature. ISRN Nanotechnology 2011:1–6. https://doi.org/10.5402/2011/483129
Sun M, Chen P, Zhao A, Zuo F (2019) Ultrasensitive detection of trinitrotoluene by Fe3O4@mTiO2/P-ATP-TNT/Au@Ag SERS sensor via synergetic effect. Anal Methods 11:1923–1929. https://doi.org/10.1039/C8AY02811G
Alizadeh T (2014) Preparation of magnetic TNT-imprinted polymer nanoparticles and their accumulation onto magnetic carbon paste electrode for TNT determination. Biosens Bioelectron 61:532–540. https://doi.org/10.1016/j.bios.2014.05.041
Zou WS, Wang YQ, Wang F, Sho Q, Zhang J, Liu J (2013) Selective fluorescence response and magnetic separation probe for 2,4,6-trinitrotoluene based on iron oxide magnetic nanoparticles. Anal Bioanal Chem 405:4905–4912. https://doi.org/10.1007/s00216-013-6873-6
USEPA (2014) Technical fact sheet–2,4,6-trinitrotoluene (TNT), Office of Solid Waste and Emergency Response, Washington, DC, USA, EPA-505-F-14-009
Sigman ME, Ma CY (2001) Detection limits for GC/MS analysis of organic explosives. Journal of Forensic Science 46:6–11. https://doi.org/10.1520/JFS14904J
Şen N, Üzek U, Aksoy Ç, Bora T, Atakol O (2015) Identification of organic explosives which have different structures by LC-MS-MS. SDU J Scien (E-Journal) 10:95–106
Ular N, Üzer A, Durmazel S, Erçağ E, Apak R (2018) Diaminocyclohexane-functionalized/Thioglycolic acid-modified gold nanoparticle-based colorimetric sensing of trinitrotoluene and tetryl. ACS Sens 3:2335–2342. https://doi.org/10.1021/acssensors.8b00709
Zhu W, Shen X, Zhu C, Li B, Hong J, Zhou X (2018) Turn-on fluorescent assay based on purification system via magnetic separation for highly sensitive probing of adenosin. Sensor Actuat B-Chem 259:855–861. https://doi.org/10.1016/j.snb.2017.12.147
Albert R, Horwitz W (1997) A heuristic derivation of the Horwitz curve. Anal Chem 69:789–790. https://doi.org/10.1021/ac9608376
Acknowledgments
The authors wish to express their gratitude to the Ministry of National Defence, Office of Technical Services, and to the Mechanical & Chemical Industry Corporation (MKEK) of Turkey for the donation of nitro and composite explosive samples. The authors extend their thanks to Istanbul University-Cerrahpaşa Research Fund (IUC BAP Unit) for the support given to Ph.D. Thesis Project–33833.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The original version of this paper was published with errors in the presentation of equations. Journal production unfortunately did not notice that the equations were incorrectly presented.
Supplementary Information
ESM 1
(DOCX 656 kb)
Rights and permissions
About this article
Cite this article
Yardımcı, B., Koç, Ö.K., Üzer, A. et al. Ethylenediamine-bound magnetite nanoparticles as dual function colorimetric sensor having charge transfer and nanozyme activity for TNT and tetryl detection. Microchim Acta 188, 228 (2021). https://doi.org/10.1007/s00604-021-04877-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-04877-z