Abstract
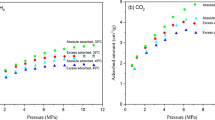
To better understand the CO2 sequestration and enhanced shale gas recovery, it is of great significance to study the adsorption characteristics of CO2 and CH4 in different types of shale. In this study, the mineral composition, pore structure and CH4 and CO2 adsorption isothermals of marine and continental shale samples were determined, an adsorption model was proposed to describe the adsorption behaviors of CH4 and CO2, the thermodynamics parameter of adsorption was obtained, and then the influence of mineral composition and pore structure on the adsorption characteristics of CH4 and CO2 in shale was clarified. The results showed that the total organic carbon content (TOC), the specific surface area (SSA) and micropore volume of marine shale samples are larger than those of continental shale samples. Shale has a higher TOC and clay minerals contents corresponding to a higher adsorption capacity. Under the same conditions, the CO2 adsorption capacity of shale is significantly higher than that of CH4. The proposed adsorption model considered the different adsorption mechanisms in different pores and the temperature effect, which can well describe the CH4 and CO2 adsorption behaviors of shale in various temperatures. Based on the adsorption model, considering the real gas conditions, the variation of the calculated isosteric heat (ΔH) and entropy (ΔS) of CH4 and CO2 adsorption with the increasing adsorption amount experienced three stages: slow decline, rapid decline, and gradual flattening. For a certain adsorption amount, the ΔH and ΔS of CO2 adsorption in shale are higher than those of CH4, and with the increase in temperature, the ΔH and ΔS show a downward trend. Combining the proposed adsorption model with ideal adsorbed solution theory, the predicted selectivity factor (\(\alpha_{{{\text{CO}}_{{2}} /{\text{CH}}_{{4}} }}\)) of CO2 over CH4 of all shale samples at the CH4 and CO2 mixed gas environment is greater than 1. Shale has a lower TOC corresponding to a higher \(\alpha_{{{\text{CO}}_{{2}} /{\text{CH}}_{{4}} }}\), and thus the \(\alpha_{{{\text{CO}}_{{2}} /{\text{CH}}_{{4}} }}\) of continental shale samples is higher than that of marine shale samples. The \(\alpha_{{{\text{CO}}_{{2}} /{\text{CH}}_{{4}} }}\) increased with the increase in fugacity and CO2 mole fraction, while decreased with the increase in temperature, and the variation of \(\alpha_{{{\text{CO}}_{{2}} /{\text{CH}}_{{4}} }}\) can be well explained by thermodynamics analysis.











Similar content being viewed by others
References
Bi, H., Jiang, Z., Li, J., Xiong, F., Li, P., Chen, L.: Ono-Kondo model for supercritical shale gas storage: a case study of Silurian Longmaxi shale in southeast Chongqing, China. Energy Fuels 31(3), 2755–2764 (2017)
Brunauer, S., Emmett, P.H., Teller, E.: Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 60(2), 309–319 (1938)
Brunauer, S., Deming, L.S., Deming, W.E., Teller, E.: On a theory of the van der Waals adsorption of gases. J. Am. Chem. Soc. 62(7), 1723–1732 (1940)
Cochran, T.W., Danner, R.P., Kabel, R.L.: Vacancy solution theory of adsorption using flory-huggins activity coefficient equations. AIChE J. 31(2), 268–277 (1985)
Curtis, J.B.: Fractured shale-gas systems. AAPG Bull 86(11), 1921–1938 (2002)
Dai, J., Zou, C., Dong, D., Ni, Y., Wu, W., Gong, D., Liu, D.: Geochemical characteristics of marine and terrestrial shale gas in China. Mar. Pet. Geol. 76, 444–463 (2016)
Dang, W., Zhang, J., Nie, H., Wang, F., Tang, X., Wu, N., Wang, R.: Isotherms, thermodynamics and kinetics of methane-shale adsorption pair under supercritical condition: Implications for understanding the nature of shale gas adsorption process. Chem. Eng. J. 383, 123191 (2020)
Deng, J., Kang, J., Zhou, F., Li, H., Zhang, D., Li, G.: The adsorption heat of methane on coal: comparison of theoretical and calorimetric heat and model of heat flow by microcalorimeter. Fuel 237, 81–90 (2019)
Du, X., Gu, M., Liu, Z., Zhao, Y., Sun, F., Wu, T.: Enhanced shale gas recovery by the injections of CO2, N2, and CO2/N2 mixture gases. Energy Fuels 33(6), 5091–5101 (2019)
Du, X., Guang, W., Cheng, Y., Hou, Z., Liu, Z., Yin, H., Shu, C.: Thermodynamics analysis of the adsorption of CH4 and CO2 on montmorillonite. Appl. Clay Sci. 192, 105631 (2020)
Duan, S., Gu, M., Du, X.D., Xian, X.F.: Adsorption equilibrium of CO2 and CH4 and their mixture on Sichuan Basin Shale. Energy Fuels 30(3), 2248–2256 (2016)
Dubinin, M.M., Astakhov, V.A.: Development of the concepts of volume filling of micropores in the adsorption of gases and vapors by microporous adsorbents. Russ. Chem. Bull. 20(1), 3–7 (1971)
Ettinger, I., Eremin, I., Zimakov, B., Yanovskaya, M.: Natural factors influencing coal sorption properties. I: Petrography and sorption properties. Fuel 45, 267–275 (1966)
Gu, M., Xian, X., Duan, S., Du, X.: Influences of the composition and pore structure of a shale on its selective adsorption of CO2 over CH4. J. Nat. Gas Sci. Eng. 46, 296–306 (2017)
Gu, M., Zhang, B., Qi, Z.D., Liu, Z.J., Duan, S., Du, X.D., Xian, X.F.: Effects of pore structure of granular activated carbons on CH4 enrichment from CH4/N2 by vacuum pressure swing adsorption. Sep. Purif. Technol. 146, 213–218 (2015)
Heller, R., Zoback, M.: Adsorption of methane and carbon dioxide on gas shale and pure mineral samples. J. Unconv. Oil Gas Resour. 8, 14–24 (2014)
Hou, Y.G., He, S., Yi, J.Z., Zhang, B.Q., Chen, X.H., Wang, Y., Cheng, C.Y.: Effect of pore structure on methane sorption potential of shales. Pet. Explor. Dev. 41(2), 272–281 (2014)
Huang, H., Li, R., Jiang, Z., Li, J., Chen, L.: Investigation of variation in shale gas adsorption capacity with burial depth: insights from the adsorption potential theory. J. Nat. Gas Sci. Eng. 73, 103043 (2020)
Ji, L., Zhang, T., Milliken, K.L., Qu, J., Zhang, X.: Experimental investigation of main controls to methane adsorption in clay-rich rocks. Appl. Geochem. 27, 2533–2545 (2012)
Kast, W.: Principles of adsorption and adsorption processes. Chem. Eng. Process. 19(2), 118–118 (1985)
Keshavarz, A., Sakurovs, R., Grigore, M., Sayyafzadeh, M.: Effect of maceral composition and coal rank on gas diffusion in Australian coals. Int. J. Coal Geol. 173, 65–75 (2017)
Langmuir, I.: The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 40(9), 1361–1403 (1918)
Li, A., Han, W., Fang, Q., Memon, A.: Ma, M: Experimental investigation of methane adsorption and desorption in water-bearing shale. Capillarity 3(3), 45–55 (2020)
Li, B., Yang, K., Ren, C., Li, J.: An adsorption-permeability model of coal with slippage effect under stress and temperature coupling condition. J. Nat. Gas Sci. Eng. 71, 102983 (2019)
Li, J., Zhou, S., Gaus, G., Li, Y., Ma, Y., Chen, K., Zhang, Y.: Characterization of methane adsorption on shale and isolated kerogen from the Sichuan Basin under pressure up to 60 MPa: Experimental results and geological implications. Int. J. Coal Geol. 189, 83–93 (2018)
Li, J.J., Yan, X.T., Wang, W.M., Zhang, Y.N., Yin, J.X., Lu, S.F., Chen, F.W., Meng, Y.L., Zhang, X.W., Chen, X., Yan, Y.X., Zhu, J.X.: Key factors controlling the gas adsorption capacity of shale: a study based on parallel experiments. Appl. Geochem 58, 88–96 (2015)
Myers, A.L., Prausnitz, J.M.: Thermodynamics of mixed-gas adsorption. AICHE J. 11(1), 121–127 (1965)
Nuttall, B.C., Eble, C.F., Drahovzal, J.A., Bustin, R.M.: Analysis of Devonian black shales in Kentucky for potential carbon dioxide sequestration and enhanced natural gas production, DE-FC26-02NT41442. Kentucky Geological Survey, Lexington, KY (2005)
Ortiz, O.P., Peredo, D., Pozo, M., Pérez, E., Bessieres, D.: Effect of organic matter and thermal maturity on methane adsorption capacity on shales from the middle magdalena valley basin in Colombia. Energy Fuels 31(11), 11698–11709 (2017)
Peng, D.Y., Robinson, D.B.: A new two-constant equation of state. Ind. Eng. Chem. Fundam. 15(1), 59–64 (1976)
Qi, R., Ning, Z., Wang, Q., Zeng, Y., Huang, L., Zhang, S., Du, H.: Sorption of methane, carbon dioxide, and their mixtures on shales from Sichuan Basin, China. Energy Fuels 32(3), 2926–2940 (2018)
Ridha, F.N., Webley, P.A.: Entropic effects and isosteric heats of nitrogen and carbon dioxide adsorption on chabazite zeolites. Micropor. Mesopor. Mat. 132, 22–30 (2010)
Ross, D.J.K., Bustin, R.M.: The importance of shale composition and pore structure upon gas storage potential of shale gas reservoirs. Mar. Pet. Geol. 26, 916–927 (2009)
Sheng, M., Li, G.S., Chen, L.J., Shao, S.J., Zhang, R.: Mechanisms analysis of shalegas supercritical adsorption and modeling of isorption adsorption. J. China Coal Soc. 39(1), 179–183 (2014)
Shi, J., Shen, G., Zhao, H., Sun, N., Song, X., Guo, Y., Sun, Y.: Porosity at the interface of organic matter and mineral components contribute significantly to gas adsorption on shales. J. CO2 Util. 28, 73–82 (2018)
Sips, R.: On the structure of a catalyst surface. J. Chem. Phys. 16(5), 490–495 (1948)
Song, X., Lü, X., Shen, Y., Guo, S., Guan, Y.: A modified supercritical Dubinin–Radushkevich model for the accurate estimation of high pressure methane adsorption on shales. Int. J. Coal Geol. 193, 1–15 (2018)
Stadie, N.P., Murialdo, M., Ahn, C.C., Fultz, B.: Anomalous isosteric enthalpy of adsorption of methane on zeolite-templated carbon. J. Am. Chem. Soc. 135(3), 990–993 (2013)
Tang, X., Ripepi, N.: High pressure supercritical carbon dioxide adsorption in coal: adsorption model and thermodynamic characteristics. J. CO2 Util. 18, 189–197 (2017)
Tang, X., Ripepi, N., Stadie, N.P., Yu, L.: Thermodynamic analysis of high pressure methane adsorption in Longmaxi shale. Fuel 193, 411–418 (2017)
Tang, X., Ripepi, N., Rigby, S., Mokaya, R.: New perspectives on supercritical methane adsorption in shales and associated thermodynamics. J. Ind. Eng. Chem. 78, 186–197 (2019)
Tian, H., Li, T., Zhang, T., Xiao, X.: Characterization of methane adsorption on overmature Lower Silurian-Upper Ordovician shales in Sichuan Basin, southwest China: experimental results and geological implications. Int. J. Coal Geol. 156, 36–49 (2016)
Wang, S., Song, Z., Cao, T., Song, X.: The methane sorption capacity of Paleozoic shales from the Sichuan Basin, China. Mar. Pet. Geol. 44, 112–119 (2013)
Weniger, P., Kalkreuth, W., Busch, A.: High-pressure methane and carbon dioxide sorption on coal and shale samples from the Parana Basin, Brazil. Int. J. Coal Geol. 84(3–4), 190–205 (2010)
Yin, H., Zhou, J., Jiang, Y., Xian, X., Liu, Q.: Physical and structural changes in shale associated with supercritical CO2 exposure. Fuel 184, 289–303 (2016)
Zhang, T., Li, Y., Sun, S.: Phase equilibrium calculations in shale gas reservoirs. Capillarity 2(1), 8–16 (2019)
Zhou, J., Hu, N., Xian, X., Zhou, L., Tang, J., Kang, Y., Wang, H.: Supercritical CO2 fracking for enhanced shale gas recovery and CO2 sequestration: results, status and future challenges. Adv. Geo-Energy Res. 3(2), 207–224 (2019)
Zhou, J., Yang, K., Tian, S., Zhou, L., Xian, X., Jiang, Y., Cai, J.: CO2-water-shale interaction induced shale microstructural alteration. Fuel 263, 116642 (2020a)
Zhou, J., Tian, S., Zhou, L., Xian, X., Yang, K., Jiang, Y., Guo, Y.: Experimental investigation on the influence of sub-and super-critical CO2 saturation time on the permeability of fractured shale. Energy 191, 116574 (2020b)
Zhou, L., Bai, S.P., Su, W., Yang, J.: Comparative study of the excess versus absolute adsorption of CO2 on super activated carbon for the near-critical region. Langmuir 19, 97–100 (2003)
Zhou, S., Ning, Y., Wang, H., Liu, H., Xue, H.: Investigation of methane adsorption mechanism on Longmaxi shale by combining the micropore filling and monolayer coverage theories. Adv. Geo-Energy Res. 2(3), 269–281 (2018a)
Zhou, S., Xue, H., Ning, Y., Guo, W.: Experimental study of supercritical methane adsorption in Longmaxi shale: Insights into the density of adsorbed methane. Fuel 211, 140–148 (2018b)
Zhou, S., Yan, G., Xue, H., Guo, W., Li, X.: 2D and 3D nanopore characterization of gas shale in Longmaxi formation based on FIB-SEM. Mar. Pet. Geol. 73, 174–180 (2016)
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (51774060, U19B2009), the Program for Changjiang Scholars and Innovative Research Team in University (IRT_17R112) and the Basic Research and Frontier Exploration Projects in Chongqing (cstc2019jcyj- msxmX0053, cstc2019yszx-jcyjX0007).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, K., Zhou, J., Xian, X. et al. Adsorption Characteristics and Thermodynamic Analysis of CH4 and CO2 on Continental and Marine Shale. Transp Porous Med 140, 763–788 (2021). https://doi.org/10.1007/s11242-021-01599-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11242-021-01599-x