Abstract
Two of the important aspects for the successful utilization of phase change materials (PCMs) for thermal energy storage systems are compatibility with container materials and stability. Therefore, the present study is focused on testing the corrosion resistance and surface characteristics of metals in contact with PCMs and thermal behavior of PCMs with heating/cooling cycles. The PCM selection is made by targeting low temperature (<100 °C) heat storage applications. The PCMs considered are paraffin wax, sodium acetate tri-hydrate, lauric acid, myristic acid, palmitic acid, and stearic acid. The metal specimens tested are aluminum, copper, and stainless steel because of their wide usage in thermal equipment. The tests are performed by the method of immersion corrosion test, and ASTM G1 standards are followed. The experiments are carried out at 80 °C and room temperature (30 °C) for the duration of 10, 30, and 60 days. Pertaining to thermal stability 1500 melting/freezing cycles are performed. Investigation has been carried out in terms of corrosion rate, SEM analysis of metal specimens, appearance of PCMs, and variation of thermophysical properties at 0th, 1000th, and 1500th thermal cycles. The most affected area of corrosion, including the dimension of pits, is presented, and comparison is made. Based on the corrosion experiments, recommendations are made for the metal–PCM pairs. Pure sodium acetate trihydrate is observed to suffer from phase segregation and supercooling. After 1500 thermal cycles, the variation in melting and freezing point temperatures for rest of the five PCMs are in the range of − 1.63 to 1.57 °C and − 4.01 to 2.66 °C. Whereas, reduction in latent heat of melting and freezing are in the range of 17.6–28.95% and 15.2–26.78%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to the rapid growth of population, improved living standard, and rapid industrialization all over the world, the energy demand in terms of conventional energy resources is rapidly increasing. The continuous exploitation of limited non-renewable reserves and a continuous increase in the level of greenhouse gas emissions drive us towards the effective utilization of renewable energy sources. Among various available renewable energy sources, solar energy can be considered as a propitious option. Due to the unpredictive and intermittent nature of solar energy, the usage of thermal energy storage systems becomes necessary for the effective utilization of solar thermal energy. As the low-temperature thermal energy storage include a wide range of applications, viz. water heating, air heating, greenhouse heating, regulating temperature in buildings etc. [1,2,3,4] a temperature range of 30–100 °C has been considered for the study. Storing of thermal energy can be achieved either using sensible or latent heat storage materials. In comparison to sensible heat storage materials, latent heat storage materials take 5–14 times less volume to store the same amount of energy [5]. The thermal energy stored/released during the phase change process is latent heat. In recent years, thermal energy storage using phase change materials has been a major topic of research, due to its large energy density and nearly isothermal heat transfer process [6, 7]. Based on the phase change process, phase change materials (PCMs) can be categorized into solid–solid, solid–liquid, solid–gas, and liquid–gas. Among these, due to high latent heat and minimum volume change, solid–liquid PCMs are preferred. Further, the solid–liquid PCMs can be classified into organic, inorganic, and eutectics [4, 8, 9].
Due to the wide range of applications, Abhat [1] considered PCMs in the temperature range of 0–120 °C. It was highlighted that inorganic salt hydrates are preferred over organics due to the higher latent heat. As inorganic PCMs suffer from supercooling, investigations were suggested prior to consideration. It was mentioned that studies pertaining to compatibility and thermal stability are necessary to assess the long term and short term usage. Zalba et al. [9] performed a comprehensive review of thermal energy storage for solid–liquid PCMs. The study was focused on materials, heat transfer, and applications. Sharma et al. [3] discussed the technology of available thermal energy storage with PCMs for different applications. Salunkhe and Krishna [4] proposed that the PCM should satisfy both primary and secondary criteria before considering for an application. It was mentioned that compatibility and thermal stability are important for efficient LHSU. As the present study focuses on two of the important aspects: compatibility and thermal stability of PCMs suitable for low-temperature heat storage applications, the literature pertaining to these studies will be discussed in the subsequent sections.
Cabeza et al. [10] considered salt hydrates PCMs namely Zn(NO3)2·6H2O, CaCl2·6H2O and Na2(HPO4)0.12H2O with melting point in 32–36 °C temperature range and tested corrosion resistance of aluminum (Al), copper (Cu), stainless steel (SS), steel and brass. Later, Cabeza et al. [11] studied corrosion of Al, Cu, SS, steel, and brass with the salt hydrate PCMs with the melting point in the range of 48–58 °C. Sodium acetate trihydrate (SAT) and sodium thiosulphate pentahydrate (STP) were considered for the study. It was suggested that with these PCMs, Al and SS can be employed, but brass and copper should be avoided. Farrell et al. [12] investigated the corrosion resistance of Al and Cu with salt hydrates. Cu showed the greatest mass loss for sodium acetate with additives. No pitting was observed on any of the Cu samples. On the other hand, all Al samples showed pitting corrosion with mass loss. García-Romero et al. [13] investigated the corrosive behavior of various aluminum alloys, namely, EN AW 2024, 3003, 6063, and 1050 in contact with Glauber’s salt. Moreno et al. [14] carried out corrosion studies for two metals and two metal alloys with salt hydrate PCMs. Recommendations were given for Cu, SS, carbon steel, and Al with 11 PCMs suitable for cooling and heating applications. Ushak et al. [15] considered MgCl2·6H2O with impurities of Salar de Atacama and studied the corrosion behavior of Cu, Al, and SS. Due to the high energy storage density, Fernandez et al. [16] considered strontium bromide and magnesium sulphate as LHSMs. Corrosion studies were performed in contact with SS, Cu, Al, and carbon steel. Carbon steel was observed to undergo corrosion with strontium bromide.
Oro et al. [17] studied the corrosion of two different families of materials, (1) metals such as Al, Cu, carbon steel, SS 316 and (2) polymer materials such polypropylene (PP), high-density polyethylene (HDPE), polyethylene terephthalate (PET) and polystyrene (PS) as container materials. The materials were tested with the selected nine PCMs of similar melting temperature of − 18 °C for cold storage application. They concluded that Cu and carbon steel must be avoided, Al is not recommended, and SS 316 is recommended when in contact with the tested PCMs. Ferrer et al. [18] considered metals viz. Al, Cu, carbon steel, SS 304, and SS 316 and investigated the corrosion rate with four PCMs. The PCMs include SP21E, PureTemp 23, CA-73.5% + MA-26.5% and CA-75.2% + PA-24.8%. Recommendations were made for metal-PCM pairs. Krishna and Kochar [19] considered Cu, Al, and SS and investigated their corrosive behavior when kept in contact with the six different PCMs suitable for low-temperature heat storage applications. SS showed to be fully compatible with the selected PCMs. Krishna and Shinde [20] measured the corrosion rate of Cu in contact with PW. The results showed that Cu is compatible with the PW. Browne et al. [21] investigated the corrosion rate of metals (Cu, Al, SS, brass and mild steel) and plastics (perspex) with five PCMs (capric acid, capric + LA, capric + PA, micronal and SP22). SS was observed to be compatible with all PCMs. But brass, Al, and Cu were recommended with caution.
Sari and kaygusuz [22] investigated the thermal hydraulics and phase change behavior of SA. Deviation in phase change behavior is noted after 10–15 heating and cooling cycles. The study was further extended to MA. The recommendation was made for the solar water heating system as it did not show any subcooling effect [23]. Sharma et al. [24] carried out thermal reliability tests on commercial grade PCMs, such as acetamide, SA, and PW. It was reported that the PCMs have not shown any degradation of the melting point after a repeated 1500 thermal cycles. Sari [25] investigated the thermal stability of selected fatty acids viz. SA, MA, LA and PA. It was noticed that these fatty acids showed good thermal stability over 0, 120, 560, 850 and 1200 number of melting/freezing cycles. Sari et al. [26] carried out synthesis, properties evaluation, and thermal reliability studies of fatty acid esters with glycerol. The PCMs showed good thermal stability for 1000 thermal cycles. Kahwaji et al. [27] considered six organic PCMs viz. decanoic acid, dodecanoic acid, tetradecanoic acid, hexadecanoic acid, octadecanoic acid, and 1-octadecanol. All the six PCMs were observed to be thermally stable for 3000 cycles, and octadecanol was compatible with all the 16 metallic alloys.
Sari and Kaygusuz [28] conducted a thermal stability test of some fatty acids viz. SA, PA, MA, and LA with melting temperatures between 40 and 63 °C. PA and MA were suggested for long term storage. SS and Al were recommended with the considered fatty acids. Dheep and Sreekumar [29] considered glutaric acid as a latent heat storage material for solar water heating, air heating, and drying applications. Studies pertaining to thermophysical properties, thermal stability for 2000 cycles, and compatibility with Cu, Al, and SS were carried out. The studies could reveal that glutaric acid is thermally stable, and SS showed a very low corrosion rate. In line with the above, Dheep and Sreekumar [30] extended the study for phenyl acetic acid. The PCM showed less corrosive nature with Al and SS when compared to Cu. To study the feasibility of acetamide as a LHSM, Brahma et al. [31] performed corrosion and thermal stability studies. The thermal stability is examined by measuring the melting point and latent heat for 1000 heating/cooling cycles. Corrosion studies were carried out with Cu, Al, SS, and mild steel. Based on the studies SS and Al were observed to be compatible with acetamide.
Several review articles highlighted the importance of compatibility and thermal stability of PCMs, and the salient discussions are as follows. Sharma and Sagara [8] presented a list of 250 PCMs with their melting point and latent heat. Studies pertaining to thermal cycles and corrosion of the materials for long term stability, methods for the enhancement of heat transfer in PCM were discussed. Vasu et al. [32] in their review article, informed that most of the organic PCMs are noncorrosive and inorganic are corrosive. SS was observed to be the most compatible material among other metals. It was suggested that Al alloy with traces of carbon is corrosive and should be avoided. Rathod and Banerjee [33] provided a detailed review discussing the thermal stability of LHSMs. Paraffins were observed to be stable after numerous heating/cooling cycles. Investigation pertaining to thermal cycling was recommended for fatty acids before consideration. Salt hydrates were noted to undergo phase segregation. Oro et al. [34] mentioned that adjustable melting/freezing point temperatures would be necessary as they are the primary selection criteria of PCM for any kind of application. Ferrer et al. [35] informed about the unavailability of common standards for performing thermal stability tests.
From the literature, it can be deduced that for low-temperature heat storage applications (30–100 °C) based on melting point temperature range, high latent heat, ease of availability and low cost, the PCMs viz. paraffin wax (PW), sodium acetate trihydrate (SAT), lauric acid (LA), myristic acid (MA), palmitic acid (PA) and stearic acid (SA) can be considered as promising contenders. For the successful latent heat storage unit (LHSU) compatibility and thermal stability are two important aspects that are to be considered. From the earlier works, ambivalence prevails pertaining to the corrosion behavior of Al, Cu, and SS with the PCMs. Also, corrosion rate, surface characteristics, and analysis of pits using SEM, the behavior of PCMs with metals at room temperature (30 °C) and above the PCMs melting point temperature (80 °C) is scarce. Pertaining to thermal reliability studies, smaller PCM volumes were taken in test tubes, where the phase segregation/subcooling effects cannot be addressed thoroughly. Besides the variation in melting point, freezing point, latent heat of melting, latent heat of freezing, and specific heat with thermal cycling tests need to be discussed. Therefore, the present study attempts to address the above issues by performing a detailed investigation of compatibility with Cu, Al, and SS and thermal stability of six PCMs (LA, MA, SA, PW, PA, and SAT). The investigation is categorized into two parts. The first part deals with the detailed compatibility studies comprising of corrosion rate and surface characteristics of metals and the appearance of PCMs. The second part deals with thermal reliability studies by performing melting and freezing cycles.
Experimental methodology
The considered six PCMs (LA, MA, SA, PW, PA, and SAT) were purchased from Hi-Media Laboratories Pvt. Ltd., India. The thermophysical properties viz. melting point, latent heat, specific heat, thermal conductivity, density, viscosity, and thermal expansion coefficient will be of great aid for the design of a LHSU. All of the above mentioned properties are experimentally evaluated and are given in Table 1.
Corrosion study
Three commonly employed container metals, namely aluminum (Al), copper (Cu), and stainless steel (SS) are selected for conducting an immersion corrosion experiment. The metal samples are prepared with dimensions of 30 mm (length) × 10 mm (width) × 2 mm (thick) for Al and SS and 1.5 mm thick for Cu. The initial appearance of the samples is shown in Fig. 1. The compositions (wt%) are Al (Al = 99.6, Fe = 0.25, Si = 0.07, Cu + Mn + Zn + Mg + Cr + Pb = 0.08), Cu (Cu = 99.85, P + Bi + Pb = 0.15) and SS (Fe = 99.2, Mn = 0.4, Si = 0.2, C = 0.2), respectively.
Methodology for corrosion
The experimental method can be divided into four steps: (1) sample preparation (2) morphology and corrosion test (3) cleaning of corroded samples and (4) evaluation of corrosion rate.
(i) Sample preparation: Initially, each of the samples is made flat with a belt grinder to achieve uniform abrasion/cleaning of the surface while polishing with hand. All the specimens are polished using hand with "silicon carbide waterproof electro-coated" abrasive papers of grades C–150, C–180, C–320, C–400, C–600, C–1000 and P–2000 in the given order gradually from coarse grain to fine grain. Initially, coarse grain paper is used for fast removal of the upper dirty layer from the metal surface so that it becomes flat easily in less time. Successively fine and finer grain abrasive papers are used to achieve a good surface finish. After marking the sample, cloth polishing is done on a disc polishing machine using a solution of 5-µm aluminum oxide powder in normal water. Once cloth polishing is over, specimens are cleaned with normal water to remove the aluminum oxide particles stuck to the surfaces. Once again cleaned with acetone followed by hot air drying. The samples are stored in airtight condition (lock cover made of polythene) to avoid interaction with the atmosphere.
(ii) Morphology and corrosion test: The corrosion method used is the immersion corrosion test. Three metals Al, Cu, and SS are tested with six PCMs, namely LA, MA, SA, PW, PA, and SAT. Before starting the corrosion experiment, the SEM (Scanning Electron Microscope) images are taken for Al, Cu, and SS at magnifications of 1000 and 4000 ×. All the prepared metal specimens are weighed using an electronic balance with the accuracy of 5 digits. The weight of each sample is recorded three times, and the average value is used for experimental purposes. The glass test tubes after cleaning with water are dried and partially filled with reference PCMs. The sample of each metal is then placed into each reference melted PCMs contained in test tubes. So that metals can get completely immersed in PCM, so the name "immersion corrosion test". Thus a total of 36 test tubes are required. To avoid further interaction with atmospheric oxygen, the tubes are covered with rubber cork. For maintaining a constant temperature (80 °C) of the tubes filled with metal–PCMs, high temperature, oil bath is used, as shown in Fig. 2. One set of 18 boiling tubes filled with metal–PCMs are arranged in the specially made fixture to hold the tubes at a proper distance so that no two test tubes can collide each other. Now the whole system of tubes with the stand is partially immersed in water of high-temperature oil bath, as shown in Fig. 2. The temperature of the oil bath is set at 80 °C, above the melting temperature of the PCMs. The other set of 18 sealed tubes containing metal–PCM (six from each of Al, Cu, and SS immersed in 6 PCMs) are kept at room temperature (below the melting temperature of PCMs) 30 °C. After 10, 30, and 60 days the metal specimens are taken out from the tubes using forceps to evaluate the corrosion rate.
(iii) Cleaning of corroded samples: PCM gets stuck with metal samples when it is taken out. To remove the PCM from the surface, the metal sample is placed in hot water, which is maintained at 80 °C. Once the PCM is removed, the sample is cleaned with water followed by chemical cleaning as per ASTM G1 standards. The cleaning is done with HNO3 (SG:1.42) for Al, HCl ((SG:1.19) with 1:1 H2O mix for the Cu submerged for 3 min, 100 ml HNO3 (SG:1.42), 20 ml HCl (SG:1.19) and reagent water to make 1000 ml solution for SS submerged for 5 min. To dry at a faster rate, the samples are exposed to hot air. Later weighing is done for the calculation of corrosion rates (i.e., gravimetric analysis). The specimens are again placed in the corresponding test tubes for 30 days. Each sample is again removed, cleaned, and reweighed. The procedure is again repeated for 60 days, and the surface characteristics are examined.
(iv) Evaluation of corrosion rate: Two methods are followed for the assessment of corrosion: (a) Gravimetric analysis and (b) SEM analysis for surface characteristics including pitting corrosion. The corrosion rate (mg/cm2 year) is estimated using Eqs. (1) and (2). The corrosion rate (CR) values obtained from calculations are compared with the help of bar charts and graphs and are discussed in the results and discussion section:
where m (t0) initial mass of the metal sample; m (t) mass of the metal sample after 10, 30 and 60 days; Δm mass loss, A area of the metal sample exposed for corrosion, and (to − t) = duration of exposure for corrosion.
Although the samples are measured before and after the test, the differences in thickness and its surface area are not significant enough to be taken into consideration. Therefore, the uniform area is used for the calculations in gravimetric analysis. Metallographic examinations are carried out with a high resolution and high magnification power scanning electron microscope (SEM) of model VEGA3-TESCAN.
Thermal stability study
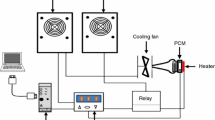
A fully automatic experimental facility is designed and fabricated for conducting the thermal cycling studies. A schematic of the experimental setup is shown in Fig. 3. The components of the experimental setup are heating bath, refrigerated cooling bath, switches for regulating power supply, data acquisition system (DAQ), and spherical containers to place PCM samples. The transparent spherical capsules are filled with the selected PCM samples and then placed inside a heating bath, which is fitted with a temperature control sensor. The power to the heaters is regulated by the DAQ to ensure the temperature of the hot water bath to be maintained at the desired value. During the cooling cycle, water from the refrigerated bath enters the heating bath via regulating the valve and flows back to the refrigerated bath. During the heating cycle, the water from the refrigerated bath is stopped by switching off the pump by the DAQ, and power supply to the heater begins until the required temperature is attained. Thus heating and cooling cycles are continued. The thermal reliability of the selected PCMs is determined by performing the melting and freezing cycles between 30 and 70 °C. These experiments are performed continuously until 500, 1000, and 1500 numbers of cycles are attained.
Results and discussions
Corrosion studies
Corrosion rate
The corrosion rate (CR) of the metal samples is calculated using Eq. (2) for the duration of 10, 30, and 60 days. It may be noted that the experiments are performed at two temperatures 30 °C and 80 °C separately. Figure 4 provides the corrosion rate (CR) versus time graph for these two temperatures. Figure 4a, b shows the CR variation with respect to time for Cu with different PCMs at 30 °C and 80 °C, respectively. Initially, for 10 days, it is maximum and observed to decrease after 30 and 60 days for all tested PCMs. The formation of a protective layer with time can be inferred for this behavior. It is clearly evident that Cu in LA at both temperatures have maximum corrosion. At 30 °C, the CR for LA is followed by PW, SAT, SA, MA, and PA, whereas at 80 °C LA is followed by SA, PA, MA, SAT, and PW. The lowest corrosion is observed in SAT and PW at 80 °C. With the increase in temperature, SAT melts and dissociates to acetate ion (CH3COO–) and sodium ion (Na+). The available water molecules in the ionic state (H+ and OH−) can lead to the formation of NaOH, which is basic in nature, causing lower CR for SAT. The high resistivity of PW due to its simple hydrocarbon chain did not cause any considerable variation of CR at 30 °C and 80 °C [19]. Figure 4c, d provides CR for Al at 30 °C and 80 °C. All the PCMs at 30 °C and PCMs other than MA and LA at 80 °C showed a decreasing trend with time. To understand the decrease and increase of CR for MA and LA, SEM images have been taken at a magnification of 4000 × and are shown in Table 2. Based on the SEM images, it is understood that severe pitting action from 30 to 60 days led to an increase in CR. Figure 4e, f provides CR for SS at 30 °C and 80 °C. The magnitude of CR for SS can be noted to be significantly less when compared with Cu and Al. The CR can be noted to be high for Cu and Al at 80 °C, but for SS, it is observed at 30 °C. As the rate of electrochemical reaction increases with temperature, higher CR is observed for Cu and Al. The formation of passive film for SS with an increase in temperature can be inferred to the drop of CR from 30 to 80 °C.
Surface morphology
SEM (scanning electron microscope) images highlighting the pits and most corroded areas are taken for the samples after 60 days of corrosion at 80 °C are given in Table 2. As there is no considerable variation of surface morphology at 30 °C, the SEM images are provided only at 80 °C. To analyze the changes caused due to corrosion, the images of metal samples are given at the 4000 × magnification. The surface morphology and dimensions of pits are highlighted and compared. The observations made for respective metal-PCM pairs are given in Table 2. In these SEM images, the pit dimensions are found to be of the order of 1–5 µm. All Al samples (at 80 °C during 60 days of corrosion) got mostly affected by pitting, whereas no pitting observed on SS samples in any PCMs. Al has the highest pitting in MA and least in PW. Cu has dominant surface corrosion in the tested PCMs, while negligible pitting observed.
PCM behavior
After 60 days, the individual test tubes kept at 80 °C and 30 °C are examined. It is observed that all the PCMs in the test tubes at 30 °C are in the solid-state except the SAT with Al and Cu. In the case of Al–SAT kept at room temperature 30 °C, the PCM remained in the liquid state. In the case of Cu–SAT kept at 30 °C, both the solid and liquid phase of SAT co-existed in the tube, i.e., some crystals of SAT formed. After 60 days, photographs of each tube (kept at 80 °C) are taken before removing the sample for the final evaluation of corrosion. Visual evaluation is done seeking bubbles, precipitates formed, surface changes, pitting in metals, and change in color of the PCMs. The images and the results found are given in Table 3. Based on the above details, it can be concluded that no visual color change is observed in PW and SAT for all three metals (Al, Cu, and SS). Cu makes all other PCMs to green–blue. Visual observations made for metal–PCM at 80 °C is summarised and given in Table 3.
Figure 5a, b shows the comparison of corrosion rates of tested metal–PCM combination kept for 60 day duration at 30 °C and 80 °C. To analyze the data obtained, the guidelines for corrosion weight loss used in the industrial sector are followed [17]. Based on the industrial guidelines, visual observation and pitting behavior, recommendations are made for the metal–PCM pairs. The recommendations based on the CR value is given in Table 4. Table 5 presents the suitability of metals with PCMs. From Fig. 5a, it can be noted that at 30 °C in the case of Cu, the lowest corrosion is found with SA followed by SAT and the highest with LA followed by PA. From Figs. 4 and 5a, it can be observed that for Cu–LA corrosion rate value is 15.43 (greater than 9.9) mg/cm2 year. Therefore, caution is recommended for Cu–LA combination. As all the other CR values are less than 9.9, they can be used without any caution for long term applications at 30 °C temperature.
From Fig. 5b, it can be observed that SS is the most corrosion resistant material at 80 °C. If Cu and Al are compared, all PCMs except MA showed higher CR with Cu. As The CR values for Cu-LA, Cu-PA, Cu-SA, and Al-MA pairs are greater than 49 mg/cm2 year, they are not recommended for service greater than 1 year. In spite of CR < 50 mg/cm2 year, due to severe pitting action as observed in Table 2, the Al-LA pair is not recommended for service greater than 1 year.
Thermal stability studies
If 300 sunny days per year are taken into consideration, and a PCM in the thermal energy storage is treated to undergo one thermal cycle (melting/freezing cycle) per day. Since a minimum life of 5 years for a thermal energy storage system is assumed, 1500 thermal cycles have been conducted for the selected PCMs. While performing corrosion experiments, it is observed that SAT did not melt completely when exposed to 80 °C and did not solidify when placed at 25 °C for several days. Therefore, prior to heating/cooling cycles, SAT (nearly 200 g) is taken in a round bottom flask (250 ml) and filled almost up to the rim to analyze the melting and solidification behavior. Figure 6a shows SAT after heating in a water bath at 80 °C for 10 h and Fig. 6b when cooled at 25 °C for 2 days. Based on Fig. 6, it can be noted that pure SAT undergoes phase segregation and cannot be employed in a LHSU.
Due to the issue of phase segregation, SAT has not been considered for thermal stability studies. To perform comprehensive thermal stability analysis, the change in thermophysical properties such as TM, TF, HM, HF, CpS, and CpL are presented after 1500 melting/freezing cycles. If the variation of the above properties is not in the acceptable range, then the PCM can be rejected for long term usage without the need for other thermophysical and chemical properties.
Paraffin wax
DSC curves of 0th, 1000th, and 1500th cycles showing heat flow variation against temperature for PW, LA, MA, PA, and SA are presented in Fig. 7, and thermal properties measured from these curves are given in Tables 6 and 7. From Table 6, TM of PW, which is initially 64.36 °C changed by − 0.59 °C after 1000 melting/freezing cycles and − 1.63 °C after 1500 melting/freezing cycles. Similarly, TF, which is initially 62.42 °C changed by 0.45 °C after 1000 cycles and 0.02 °C after 1500 cycles. Based on Table 6 it can be noticed that the HM for PW changed by − 0.48% and − 26.28% and HF by 0.27% and − 25.93%, respectively, after 1000 and 1500 cycles. These results show that considerable variation in latent heat (HM and HF) is observed after 1500 cycles. From these results, it can be noted that up to 1000 cycles, PW is the suitable PCM as the variation of TM and TF are less than 1 °C, and the change in HM and HF is within 0.5%. Further, variation in specific heat at 0th and after the 1000th and 1500th thermal cycles are given in Table 7. From these results, the degradation in specific heat (solid and liquid) values can be noted. The variation in the specific heat of solid PW is observed to be − 5.14% and 11.68% and liquid PW by 3.81% and − 7.61% after 1000 and 1500 thermal cycles.
Lauric acid
The phase change temperatures (TM and TF) and latent heat (HF and HM) of the un-cycled LA (at 0th cycle) are presented in Table 6. DSC curves are measured (for both heating and cooling) for 0th, 1000th, and 1500th thermal cycles and are given in Fig. 7. From these DSC curves, one can observe the behavior of these properties with the number of melting/freezing cycles. The details pertaining to this are presented in Tables 6 and 7. From these results, variations of 1.56 °C and 1.57 °C are noticed in the melting point temperature and, 1.28 °C and 2.46 °C variations are observed in freezing point temperature after 1000 and 1500 thermal cycles. As given in Table 6, the latent heat of melting reduced by 13.69% (at 1000th cycle) and 23.27% (at 1500th cycle) with respect to the zero cycle values. In line with this the reduction in latent heat of freezing values is noted to be 11.38% after the 1000th cycle and 20.22% after the 1500th cycle. These results convey that variations of latent heat (HM and HF) with the increase in the number of melting/freezing cycles are not regular. Table 7 deduces the effect of repeated thermal cycles on the stability of the LA in terms of change in its specific heat (solid and liquid). As shown in Table 7, no significant degradation in the specific heat of LA is observed.
Myristic acid
The TM, TF, HM and HF for MA are measured at 0th, 1000th, and 1500th melting/freezing cycles, and their values are given in Table 6. The DSC curves of MA for considered melting/freezing cycles are shown in Fig. 7. As shown in Fig. 7 and Table 6, the variation in melting and freezing point temperatures are not too high. TM has a variation of 1.41 °C (at 1000 thermal cycles) and 1.46 °C (at 1500 thermal cycles) when compared to the values of the zeroth cycle. Whereas, TF has a variation of 3 °C (at 1000 thermal cycles) and 2.6 °C (at 1500 thermal cycles). As shown in Table 6, HM for myristic acid decreased by 18.9% and 25.84% and HF by 6.9% and 15.2% after the repeated 1000 and 1500 thermal cycles. Form these results; it can be noticed that HM and HF of the MA got reduced with the increase in the number of melting/freezing cycles. On the other hand, the variation in Cp (solid and liquid) is given in Table 7. After 1500 thermal cycles, the Cp (solid and liquid) values of the zeroth thermal cycle are almost repeated. This infers that no significant variation in the specific heat values during the melting and freezing cycles.
Palmitic acid
DSC curves measured for PA at 0th and after 1000th and 1500th thermal cycles are presented in Fig. 7, and the thermal properties are given in Table 6. As shown in Table 6 and Fig. 7, no significant variation in TM and TF are observed from 1000 to 1500 cycles. TM of PA showed a variation of 1.43 °C after the 1000 cycles and 1.39 °C after 1500 cycles. Similarly, TF changed by 2.32 °C and 1.89 °C after 1000 and 1500 thermal cycles. On the other hand, significant variation in latent heat (HM and HF) is observed during the thermal cycle test. As shown in Table 6, after 1000 and 1500 cycles, HM displayed a variation of − 19.4% and − 17.6% and HF by − 18.73% and − 16.18%, respectively. These results indicate a decrease in latent heat (HM and HF) of PA with thermal cycles. It can also be observed that these values are almost constant after 1000 cycles. Subsequently, the obtained specific heat of PA at 0th and after 1000 and 1500 cycles are given in Table 7. As given in Table 7, the specific heat of solid PCM after 1000 and 1500 cycles varies by − 13.79% and 0.49%, while the specific heat of liquid PCM decreased by − 12.1% and − 22.65%, respectively. These results showed that the variation in the specific heat (solid and liquid) of the PA with the number of melting/freezing cycles is irregular.
Stearic acid
For the thermal stability of SA, a similar process has been pursued. The thermal cycle test for 1500 melting/freezing cycles is carried out, and the DSC measurements are recorded at the starting and after 1000 and 1500 cycles. The DSC curves are given in Fig. 7, the corresponding thermophysical properties are provided in Table 6. From Table 6, it can be noticed that no significant deviation in TM is observed during the thermal cycling process. The TM value varied by 0.75 °C and 1.12 °C, whereas TF varied by − 3.91 °C and − 4.01 °C for 1000 and 1500 thermal cycles. From Fig. 7 and Table 6, it can be noticed that the HM and HF of SA show considerable degradation with the increasing number of melting/freezing cycles. After repeated 1000 and 1500 melting/freezing cycles, the variation in the HM is − 17.69% and − 28.95%, respectively, and the variation in HF is − 15.7% and − 26.78%. The degradation in the latent heat (both HM and HF) may be due to the impurities or changes in the chemical structure of SA. Furthermore, Table 7 describes the effect of melting/freezing cycles on specific heat (solid and liquid states) of the SA. Based on these results, it is found that the specific heat of solid PCM varies by 1.51–3.5% and liquid PCM by − 1.68 to − 2.11% after 1000 and 1500 thermal cycles. From the earlier studies [28, 36] it can be observed that the variation of thermal properties of SA is at an acceptable level to apply as latent heat storage material.
Conclusions
The study presents thermal stability and compatibility of container materials (Cu, Al, and SS) with promising PCMs suitable for low temperature (< 100 °C) thermal energy storage. Six PCMs viz. paraffin wax, sodium acetate trihydrate, lauric acid, myristic acid, palmitic acid, and stearic acid and three metals are considered for the study. The corrosion rate is observed for the duration of 10, 30, and 60 days and thermal stability for 1000 and 1500 heating/cooling cycles. The thermophysical properties of these PCMs are measured and tabulated. Based on the investigation, the following conclusions can be drawn.
Corrosion studies
-
The corrosion rate of copper is high when compared to aluminum. Insignificant mass loss is noted for stainless steel.
-
Copper is observed to undergo surface corrosion and aluminum pitting corrosion.
-
The combination of copper with lauric acid, palmitic acid, and stearic acid should be avoided due to the higher surface corrosion.
-
The combination of aluminum with myristic acid and lauric acid should be avoided due to severe pitting action. The pitting corrosion will cause the formation of holes in the components, resulting in the failure of the system.
-
The corrosion rate is more for copper and aluminum at 80 °C, whereas for stainless steel, it is observed at 30 °C.
-
Initially, the corrosion rate for copper and aluminum is high for all the combinations, as time progresses, the corrosion rate continuously decreased with the exception of aluminum–lauric acid and aluminum–myristic acid.
-
At 80 °C, paraffin wax and sodium acetate trihydrate can be a promising option among the tested PCMs as there was no significant corrosion observed for all the three metals (Al, Cu, and SS).
-
Stainless steel can be employed with any of the six PCMs as it is found to be the most corrosion resistant material at 30 °C and 80 °C.
Thermal stability studies
-
Pure sodium acetate trihydrate cannot be used for thermal energy storage due to phase segregation and supercooling.
-
After 1500 thermal cycles, the maximum variation for the melting point is observed to be − 1.63 °C for paraffin wax, and the maximum variation for the freezing point is − 4.01 °C for stearic acid.
-
The enthalpy of melting and freezing of the selected organic PCMs decreased with the increase in the number of repeated melting/freezing cycles.
-
Stearic acid is noted to undergo a maximum reduction of 28.95% for the latent heat of melting and 26.78% for the latent heat of freezing.
-
The performance of a latent heat storage unit depends on both melting and solidification phenomena with stable/minimum variation in both melting and freezing temperatures. Among all the selected PCMs, if both melting point and freezing point are considered paraffin wax is observed to be stable with a deviation of − 1.63 °C for melting point and 0.02 °C for the freezing point.
-
Pertaining to specific heat, palmitic acid is observed to attain a maximum variation of − 22.66%.
-
The variation of specific heat values for both solid and liquid states is observed to be less than 1% for lauric acid and myristic acid.
-
Typically, a variation of ± 5 °C for PCM’s melting point and freezing point temperatures is acceptable. Based on which the considered PCMs can be treated as an attractive option for latent heat storage.
Abbreviations
- CR:
-
Corrosion rate
- \(C_{{\text{p}}}\) :
-
Specific heat, J/(kg K)
- H :
-
Latent heat, J/kg
- T :
-
Temperature, °C
- \(\beta\) :
-
Thermal expansion coefficient, K−1
- \(\mu\) :
-
Dynamic viscosity, kg/m-s
- \(\rho\) :
-
Density, kg/m3
- Avg:
-
Average
- F:
-
Freezing
- L:
-
Liquid
- M:
-
Melting
- S:
-
Solid
- LA:
-
Lauric acid
- LHSU:
-
Latent heat storage unit
- MA:
-
Myristic acid
- PA:
-
Palmitic acid
- PCM:
-
Phase change material
- PW:
-
Paraffin wax
- SA:
-
Stearic acid
- SAT:
-
Sodium acetate trihydrate
References
Abhat, A.: Low temperature latent heat thermal energy storage: heat storage materials. Sol. Energy 30, 313–332 (1983). https://doi.org/10.1016/0038-092X(83)90186-X
Kalapala, L., Devanuri, J.K.: Influence of operational and design parameters on the performance of a PCM based heat exchanger for thermal energy storage: a review. J. Energy Storage 20, 497–519 (2018). https://doi.org/10.1016/j.est.2018.10.024
Sharma, A., Tyagi, V.V., Chen, C.R., Buddhi, D.: Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 13, 318–345 (2009). https://doi.org/10.1016/j.rser.2007.10.005
Salunkhe, P.B., Jaya Krishna, D.: Investigations on latent heat storage materials for solar water and space heating applications. J. Energy Storage 12, 243–260 (2017). https://doi.org/10.1016/j.est.2017.05.008
Kalapala, L., Devanuri, J.K.: Parametric investigation to assess the melt fraction and melting time for a latent heat storage material based vertical shell and tube heat exchanger. Sol. Energy 193, 360–371 (2019). https://doi.org/10.1016/j.solener.2019.09.076
Sardari, P.T., Giddings, D., Grant, D., Gillott, M., Walker, G.S.: Discharge of a composite metal foam/phase change material to air heat exchanger for a domestic thermal storage unit. Renew. Energy. 148, 987–1001 (2020). https://doi.org/10.1016/j.renene.2019.10.084
Kalapala, L., Devanuri, J.K.: Optimization of fin parameters to reduce entropy generation and melting time of a latent heat storage unit. J. Sol. Energy Eng. (2020). https://doi.org/10.1115/1.4046878
Sharma, S.D., Sagara, K.: Latent heat storage materials and systems: a review. Int. J. Green Energy 2(1), 56 (2005). https://doi.org/10.1081/ge-200051299
Zalba, B., Marín, J.M., Cabeza, L.F., Mehling, H.: Review on thermal energy storage with phase change: materials, heat transfer analysis and applications. Appl. Therm. Eng. 23, 251–283 (2003). https://doi.org/10.1016/S1359-4311(02)00192-8
Cabeza, L.F., Illa, J., Roca, J., Badia, F., Mehling, H., Hiebler, S., Ziegler, F.: Middle term immersion corrosion tests on metal–salt hydrate pairs used for latent heat storage in the 32–36 °C temperature range. Mater. Corros. 52, 748 (2001). https://doi.org/10.1002/1521-4176(200110)52:10%3c748::AID-MACO748%3e3.0.CO;2-S
Cabeza, L.F., Roca, J., Nogués, M., Mehling, H., Hiebler, S.: Immersion corrosion tests on metal–salt hydrate pairs used for latent heat storage in the 48–58 °C temperature range. Mater. Corros. 53, 902–907 (2002). https://doi.org/10.1002/maco.200290004
Farrell, A.J., Norton, B., Kennedy, D.M.: Corrosive effects of salt hydrate phase change materials used with aluminium and copper. J. Mater. Process. Technol. 175, 198–205 (2006). https://doi.org/10.1016/j.jmatprotec.2005.04.058
Garcia-Romero, A., Delgado, A., Urresti, A., Martin, K., Sala, J.M.: Corrosion behaviour of several aluminium alloys in contact with a thermal storage phase change material based on Glauber’s salt. Corros. Sci. 51, 1263–1272 (2009). https://doi.org/10.1016/j.corsci.2009.03.006
Moreno, P., Miró, L., Solé, A., Barreneche, C., Solé, C., Martorell, I., Cabeza, L.F.: Corrosion of metal and metal alloy containers in contact with phase change materials (PCM) for potential heating and cooling applications. Appl. Energy 125, 238–245 (2014). https://doi.org/10.1016/j.apenergy.2014.03.022
Ushak, S., Marín, P., Galazutdinova, Y., Cabeza, L.F., Farid, M.M., Grágeda, M.: Compatibility of materials for macroencapsulation of inorganic phase change materials: experimental corrosion study. Appl. Therm. Eng. 107, 410–419 (2016). https://doi.org/10.1016/j.applthermaleng.2016.06.171
Fernández, A.G., Fullana, M., Calabrese, L., Palomba, V., Frazzica, A., Cabeza, L.F.: Corrosion assessment of promising hydrated salts as sorption materials for thermal energy storage systems. Renew. Energy 150, 428–434 (2020). https://doi.org/10.1016/j.renene.2020.01.001
Oró, E., Miró, L., Barreneche, C., Martorell, I., Farid, M.M., Cabeza, L.F.: Corrosion of metal and polymer containers for use in PCM cold storage. Appl. Energy 109, 449–453 (2013). https://doi.org/10.1016/j.apenergy.2012.10.049
Ferrer, G., Sole, A., Barreneche, C., Martorell, I., Cabeza, L.F.: Corrosion of metal containers for use in PCM energy storage. Renew. Energy 76, 465–469 (2015). https://doi.org/10.1016/j.renene.2014.11.036
Jaya Krishna, D., Kochar, S.: The metallographic study of corrosion of metals with latent heat storage materials suitable for solar hot water system. Trans. Indian Ceram. Soc. 76, 133–141 (2017). https://doi.org/10.1080/0371750X.2016.1268072
Krishna, D.J., Shinde, A.: Step by step methodology for the assessment of metal corrosion rate with PCMs suitable for low temperature heat storage applications. Mater. Today Proc. 4, 10039–10042 (2017). https://doi.org/10.1016/j.matpr.2017.06.317
Browne, M.C., Boyd, E., McCormack, S.J.: Investigation of the corrosive properties of phase change materials in contact with metals and plastic. Renew. Energy 108, 555–568 (2017). https://doi.org/10.1016/j.renene.2017.02.082
Sari, A., Kaygusuz, K.: Thermal energy storage system using stearic acid as a phase change material. Sol. Energy 71, 365–376 (2001). https://doi.org/10.1016/S0038-092X(01)00075-5
Sarı, A., Kaygusuz, K.: Thermal performance of myristic acid as a phase change material for energy storage application. Renew. Energy 24, 303–317 (2001). https://doi.org/10.1016/S0960-1481(00)00167-1
Sharma, A., Sharma, S., Buddhi, D.: Accelerated thermal cycle test of acetamide, stearic acid and paraffin wax for solar thermal latent heat storage applications. Energy Convers. Manag. 43, 1923–1930 (2002). https://doi.org/10.1016/S0196-8904(01)00131-5
Sarı, A.: Thermal reliability test of some fatty acids as PCMs used for solar thermal latent heat storage applications. Energy Convers. Manag. 44, 2277–2287 (2003). https://doi.org/10.1016/S0196-8904(02)00251-0
Sarı, A., Biçer, A., Karaipekli, A., Alkan, C., Karadag, A.: Synthesis, thermal energy storage properties and thermal reliability of some fatty acid esters with glycerol as novel solid–liquid phase change materials. Sol. Energy Mater. Sol. Cells 94, 1711–1715 (2010). https://doi.org/10.1016/j.solmat.2010.05.033
Kahwaji, S., Johnson, M.B., Kheirabadi, A.C., Groulx, D., White, M.A.: Fatty acids and related phase change materials for reliable thermal energy storage at moderate temperatures. Sol. Energy Mater. Sol. Cells 167, 109–120 (2017). https://doi.org/10.1016/j.solmat.2017.03.038
Sari, A., Kaygusuz, K.: Some fatty acids used for latent heat storage: thermal stability and corrosion of metals with respect to thermal cycling. Renew. Energy 28, 939–948 (2003). https://doi.org/10.1016/S0960-1481(02)00110-6
Dheep, G.R., Sreekumar, A.: Investigation on thermal reliability and corrosion characteristics of glutaric acid as an organic phase change material for solar thermal energy storage applications. Appl. Therm. Eng. 129, 1189–1196 (2018). https://doi.org/10.1016/j.applthermaleng.2017.10.133
Raam Dheep, G., Sreekumar, A.: Thermal reliability and corrosion characteristics of an organic phase change materials for solar space heating applications. J. Energy Storage 23, 98–105 (2019). https://doi.org/10.1016/j.est.2019.03.009
Brahma, B., Narzary, R., Baruah, D.C.: Acetamide for latent heat storage: thermal stability and metal corrosivity with varying thermal cycles. Renew. Energy 145, 1932–1940 (2020). https://doi.org/10.1016/j.renene.2019.07.109
Vasu, A., Hagos, F.Y., Noor, M.M., Mamat, R., Azmi, W.H., Abdullah, A.A., Ibrahim, T.K.: Corrosion effect of phase change materials in solar thermal energy storage application. Renew. Sustain. Energy Rev. 76, 19–33 (2017). https://doi.org/10.1016/j.rser.2017.03.018
Rathod, M.K., Banerjee, J.: Thermal stability of phase change materials used in latent heat energy storage systems: a review. Renew. Sustain. Energy Rev. 18, 246–258 (2013). https://doi.org/10.1016/j.rser.2012.10.022
Oró, E., Gracia, A.D., Castell, A., Farid, M.M., Cabeza, L.F.: Review on phase change materials (PCMs) for cold thermal energy storage applications. Appl. Energy 99, 513–533 (2012). https://doi.org/10.1016/j.apenergy.2012.03.058
Ferrer, G., Solé, A., Barreneche, C., Martorell, I., Cabeza, L.F.: Review on the methodology used in thermal stability characterization of phase change materials. Renew. Sustain. Energy Rev. 50, 665–685 (2015). https://doi.org/10.1016/j.rser.2015.04.187
Sharma, A., Shukla, A.: Thermal cycle test of binary mixtures of some fatty acids as phase change materials for building applications. Energy Build. 99, 196–203 (2015). https://doi.org/10.1016/j.enbuild.2015.04.028
Acknowledgements
The authors would like to thank SERB—Fast Track Scheme for Young Scientists, Department of Science and Technology (DST), Government of India for funding this research work (no.: SB/FTP/ETA-0130/2014).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Devanuri, J.K., Gaddala, U.M. & Kumar, V. Investigation on compatibility and thermal reliability of phase change materials for low-temperature thermal energy storage. Mater Renew Sustain Energy 9, 24 (2020). https://doi.org/10.1007/s40243-020-00184-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40243-020-00184-4