Abstract
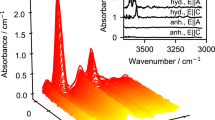
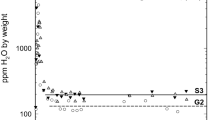
We performed in situ infrared spectroscopic measurements of OH bands in a forsterite single crystal between −194 and 200 °C. The crystal was synthesized at 2 GPa from a cooling experiment performed between 1,400 and 1,275 °C at a rate of 1 °C per hour under high silica-activity conditions. Twenty-four individual bands were identified at low temperature. Three different groups can be distinguished: (1) Most of the OH bands between 3,300 and 3,650 cm−1 display a small frequency lowering (<4 cm−1) and a moderate broadening (<10 cm−1) as temperature is increased from −194 to 200 °C. The behaviour of these bands is compatible with weakly H-bonded OH groups associated with hydrogen substitution into silicon tetrahedra; (2) In the same frequency range, two bands at 3,617 and 3,566 cm−1 display a significantly anharmonic behaviour with stronger frequency lowering (42 and 27 cm−1 respectively) and broadening (~30 cm−1) with increasing temperature. It is tentatively proposed that the defects responsible for these OH bands correspond to H atoms in interstitial position; (3) In the frequency region between 3,300 and 3,000 cm−1, three broad bands are identified at 3,151, 3,178 and 3,217 cm−1, at −194 °C. They exhibit significant frequency increase (~20 cm−1) and broadening (~70 cm−1) with increasing temperature, indicating moderate H bonding. These bands are compatible with (2H)Mg defects. A survey of published spectra of forsterite samples synthesized above 5 GPa shows that about 75 % of the incorporated hydrogen belongs to type (1) OH bands associated with Si substitution and 25 % to the broad band at 3,566 cm−1 (type (2); 3,550 cm−1 at room temperature). The contribution of OH bands of type (3), associated to (2H)Mg defects, is negligible. Therefore, solubility of hydrogen in forsterite (and natural olivine compositions) cannot be described by a single solubility law, but by the combination of at least two laws, with different activation volumes and water fugacity exponents.







Similar content being viewed by others
References
Bai Q, Kohlstedt DL (1993) Substantial hydrogen solubility in olivine and implications for water storage in the mantle. Nature 357:672–674
Balan E, Refson K, Blanchard M, Delattre S, Lazzeri M, Ingrin J, Mauri F, Wright K, Winkler B (2008) Theoretical infrared absorption coefficient of OH groups in minerals. Am Mineral 93:950–953
Balan E, Delattre S, Guillaumet M, Salje EKH (2010) Low-temperature infrared spectroscopic study of OH stretching modes in kaolinite and dickite. Am Mineral 95:1257–1266
Balan E, Ingrin J, Delattre M, Kovacs I, Blanchard M (2011) Theoretical infrared spectrum of OH defects in forsterite. Eur J Miner 23:285–292
Bali E, Bolfan-Casanova N, Koga KT (2008) Pressure and temperature dependence of H solubility in forsterite: an implication to water activity in the Earth interior. Earth Planet Sci Lett 268:354–363
Bell DR, Rossman GR, Maldener J, Endisch D, Rauch F (2003) Hydroxide in olivine: a quantitative determination of the absolute amount and calibration of the IR spectrum. J Geophys Res 108:2105–2113
Berry AJ, Hermann J, O’Neill HC, Foran GJ (2005) Fingerprinting the water site in mantle olivine. Geology 33:869–872
Berry AJ, O’Neill HC, Hermann J, Scott DR (2007) The infrared signature of water associated with trivalent cations in olivine. Earth Planet Sci Lett 261:134–142
Blanchard M, Balan E, Wright K (2009) Incorporation of water in iron-free ringwoodite: a first-principles study. Am Mineral 94:83–89
Braithwaite JS, Wright K, Catlow CRA (2003) A theoretical study of the energetics and IR frequencies of hydroxyl defects in forsterite. J Geophys Res 108:2284
Brodholt JP, Refson K (2000) An ab initio study of hydrogen in forsterite and a possible mechanism for hydrolytic weakening. J Geophys Res 105:18977–18982
Cardona M, Ruf T (2001) Phonon self-energies in semiconductors: anharmonic and isotopic contributions. Solid State Commun 117:201–212
Grant KJ, Kohn SC, Brooker RA (2006) Solubility and partitioning of water in synthetic forsterite and enstatite in the system MgO–SiO2–H2O ± Al2O3. Contrib Mineral Petrol 151:651–664
Grant KJ, Kohn SC, Brooker RA (2007a) The partitioning of water between olivine, orthopyroxene and melt synthesised in the system albite–forsterite–H2O. Earth Planet Sci Lett 260:227–241
Grant KJ, Brooker RA, Kohn SC, Wood BJ (2007b) The effect of oxygen fugacity on hydroxyl concentrations and speciation in olivine: implications for water solubility in the upper mantle. Earth Planet Sci Lett 261:217–229
Hirth G, Kohlstedt DL (1996) Water in the oceanic upper mantle: implications for rheology, melt extraction and the evolution of the lithosphere. Earth Planet Sci Lett 144:93–108
Hushur A, Manghnani MH, Smyth JR, Nestola F, Frost DJ (2009) Crystal chemistry of hydrous forsterite and its vibration properties up to 41 GPa. Am Mineral 94:751–760
Jung H, Karato S (2001) Water-induced fabric transitions in olivine. Science 293:1460–1463
Karato S (2006) Influence of hydrogen-related defects on the electrical conductivity and plastic deformation of mantle minerals: a critical review. In: Jacobsen SD, Van der Lee S (eds) Earth’s deep water cycle. American Geophysical Union, Washington, pp 113–129
Katayama I, Karato S (2008) Low-temperature, high-stress deformation of olivine under water-saturated conditions. Phys Earth Planet Int 168:125–133
Kohlstedt DL (2006) The role of water in High-temperature rock-deformation. In: Keppler H, Smyth JR (eds) Water in nominally anhydrous minerals. Miner Soc Am and Geochem Soc, Rev Mineral Geochem 62:377–396
Kohlstedt DL, Keppler H, Rubie DC (1996) Solubility of water in the a, b and g phases of (Mg, Fe)2SiO4. Contrib Mineral Petrol 123:345–357
Kovacs I, O’Neill HSC, Hermann J, Hauri EH (2010) Site-specific infrared O–H absorption coefficients for water substitution into olivine. Am Mineral 95:292–299
Kudoh Y, Kuribayashi T, Kagi H, Inoue T (2006) Cation vacancy and possible hydrogen positions in hydrous forsterite, Mg1.985Si0.993H0.06O4, synthesized at 13.5 GPa and 1,300 °C. J Mineral Petrol Sci 101:265–269
Lager GA, Armbruster TH, Faber J (1987) Neutron and X-ray diffraction study of hydrogarnet Ca3Al2(O4H4)3. Am Mineral 72:756–765
Lemaire C, Kohn SC, Brooker RA (2004) The effect of silica activity on the incorporation mechanism of water in synthetic forsterite: a polarised infrared spectroscopic study. Contrib Mineral Petrol 147:48–57
Libowitzky E (1999) Correlation of O–H stretching frequencies and O–H···O hydrogen bond lengths in minerals. Monatsh Chem 130:1047–1059
Libowitzky E, Rossman GR (1997) An IR absorption calibration for water in minerals. Am Mineral 82:1111–1115
Litasov KD, Ohtani E, Kagi H, Jacobsen SD (2007) Temperature dependence and mechanism of hydrogen incorporation in olivine at 12.5–14.0 GPa. Geophys Res Lett 34:L16314
Lüpke G, Tolk NH, Feldman LC (2003) Vibrational lifetimes of hydrogen in silicon. J Appl Phys 93:2317–2323
Martin KR, Blaney P, Shi G, Stavola M, Fowler WB (2006) Temperature dependence of the vibrational spectrum of a Li–OH complex in ZnO: infrared absorption experiments and theory. Phys Rev B 73:232509. doi:10.1103/PhysRevB.73.235209
Matveev S, O’Neill HC, Ballhaus C, Taylor WR, Green DH (2001) Effect of silica activity on OH–IR spectra of olivine: implications for low-aSiO2 mantle metasomatism. J Petrol 42:721–729
Matveev S, Portnyagin M, Ballhaus R, Brooker R, Geiger CA (2005) FTIR spectrum of phenocryst olivine as an indicator of silica saturation in magmas. J Petrol 46:603–614
Mei S, Kohlstedt DL (2000a) Influence of water on plastic deformation of olivine aggregates (1): diffusion creep regimes. J Geophys Res 105:21457–21469
Mei S, Kohlstedt DL (2000b) Influence of water on plastic deformation of olivine aggregates (2): dislocation creep regimes. J Geophys Res 105:21471–21481
Mosenfelder JL, Deligne NI, Asimow PD, Rossman GR (2006) Hydrogen incorporation in olivine from 2–12 GPa. Am Mineral 91:285–294
Mosenfelder JL, Le Voyer M, Rossman GR, Guan Y, Bell DR, Asimow PD, Eiler JM (2011) Analysis of hydrogen in olivine by SIMS: evaluation of standards and protocol. Am Mineral 96:1725–1741
Novak GA, Gibbs GV (1971) The crystal chemistry of the silicate garnets. Am Mineral 56:791–825
Otsuka K, Karato S (2011) Control of the water fugacity at high pressures and temperatures: applications to the incorporation mechanisms of water in olivine. Phys Earth Planet Int 189:27–33
Rossman GR, Aines RD (1991) The hydrous components in garnets: grossular–hydrogrossular. Am Mineral 76:1153–1164
Seko A, Oba F, Kuwabara A, Tanaka I (2005) Pressure-induced phase transition in ZnO and ZnO–MgO pseudobinary system: a first-principles lattice dynamics study. Phys Rev B 72:024107. doi:10.1103/PhysRevB.72.024107
Shaw DM, Tse JS (2007) Vibrational dynamics of H+-substituted forsterite: a first-principles molecular dynamics study. Am Mineral 92:1593–1600
Smyth JR, Frost DJ, Nestola F, Holl CM, Bromiley G (2006) Olivine hydration in the deep upper mantle: effects of temperature and silica activity. Geophys Res Lett 33:L15301
Umemoto K, Wentzcovitch RM, Hirschmann MM, Kohlstedt DL, Withers AC (2011) A first-principles investigation of hydrous defects and IR frequencies in forsterite: the case for Si vacancies. Am Mineral 96:1475–1479
Verma AK, Karki BB (2009) Ab initio investigations of native and protonic point defects in Mg2SiO4 polymorphs under high pressure. Earth Planet Sci Lett 285:140–149
Walker AM, Wright K, Slater B (2003) A computational study of oxygen diffusion in olivine. Phys Chem Miner 30:536–545
Walker AM, Demouchy S, Wright K (2006) Computer modelling of the energies and vibrational properties of hydroxyl groups in a and b-Mg2SiO4. Eur J Miner 18:529–543
Walker AM, Hermann J, Berry AJ, O’Neill HSC (2007) Three water sites in upper mantle olivine and the role of titanium in the water weakening mechanism. J Geophys Res 112:B05211
Wang Q (2010) A review of water contents and ductile deformation mechanisms of olivine: implications for the lithosphere-asthenosphere boundary of continents. Lithos 120:30–41
Withers AC, Hirschmann MM, Tenner TJ (2011) The effect of Fe on olivine H2O storage capacity: consequences for H2O in the Martian mantle. Am Mineral 96:1039–1053
Withers AC, Bureau H, Raepsaet C, Hirschmann MM (2012) Calibration of infrared spectroscopy by elastic recoil detection analysis of H in synthetic olivine. Chem Geol 334:92–98
Zhao YH, Ginsberg SB, Kohlstedt DL (2004) Solubility of hydrogen in olivine: dependence on temperature and iron content. Contrib Mineral Petrol 147:155–161
Acknowledgments
This work was supported by the ANR grant (NT09-566853) provided to J. Ingrin. We thank Jed Mosenfelder and an anonymous reviewer for useful comments to the manuscript. We thank EU for the funding of Liu Jia during his stay in Lille through the LiSUM project from the Erasmus Mundus External Cooperation Window program.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ingrin, J., Liu, J., Depecker, C. et al. Low-temperature evolution of OH bands in synthetic forsterite, implication for the nature of H defects at high pressure. Phys Chem Minerals 40, 499–510 (2013). https://doi.org/10.1007/s00269-013-0587-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-013-0587-3