Abstract
Multicomponent nanoframes (NFs) with a hollow structural character have shown the potential to be applied in many fields. Here we report a novel strategy to synthesize ZnxCd1−xS NFs via the synergistic actions of the graphene oxide (GO) confinement effect and oriented cation exchange. The obtained samples have been systematically characterized by x-ray diffractometry (XRD), field-emission scanning electron microscopy (SEM), transmission electron microscopy (TEM), x-ray photospectroscopy (XPS) and Raman spectrometry. The results show that the two dimensional space confinement effect induced by GO and the oriented cation exchange reaction are responsible for the formation of the multicomponent NFs. The high photoelectrochemical activity and the low cost of the starting materials will make the multicomponent NFs applicable in photoelectronic and photoelectrocatalytic fields.
Export citation and abstract BibTeX RIS
1. Introduction
Hollow nanostructures have attracted a great deal of research attention due to their superior performance over their solid counterparts with regard to energy conversion [1, 2], storage [3, 4], and sensing [5]. Compared to the conventional hollow nanostructures, such as nanocoils [5, 6], nanorings [7], nanoskeletons [8], and nanocages [1], nanoframes (NFs) are recently developed hollow structures and possess excellent properties [9–14]. Various routes including self-assembly, oriented attachment and template-assisting [8] have been developed to obtain NFs. Nevertheless, complex synthetic procedures and rigorous experimental conditions are always needed. Furthermore, the synthesis of NFs with designed compositions is still a great challenge.
Recently, Zhang et al reported the synthesis of NFs via the selective cation-exchange reaction of unpurified inorganic–organic hybrid nanosheets (NSs) [9]. It is demonstrated that the cation exchange reaction is an effective route to obtain hollow NF structures with controlled composition. However, the final products are determined by the cleanliness of the precursor and have limitations to some degree because the surface chemical environment of the unpurified hybrid NSs is the key for the formation of NFs. On the other hand, various nanoparticle-decorated graphene composites have been prepared to increase photoelectronic and catalytical performance [15, 16]. Nevertheless, the agglomeration of the supported nanoparticles caused by their large surface/interface energy and structural disorder inevitably degrades their properties rapidly. In this regard, NFs assembled by nanoparticles as typical hierarchical micro/nano structures can reduce the agglomeration effectively. Recently, we demonstrated that GO has a space confinement effect, which means GO can construct a quasi-2D space [17]. This space effectively controlled the evolution of solid NSs to hollow NFs. The novel mechanism successfully promoted the formation of composite of ZnS NF and reduced graphene oxide (ZnS-NF@rGO). However, to the best of our knowledge, until now there has been no report on the synthesis of multicomponent NFs@rGO composites.
Herein, taking both merits of the GO space confinement effect and the oriented cation exchange reaction, we have successfully synthesized NFs wrapped by rGO with tunable compositions for the first time. As a proof of concept, ZnxCd1−xS-NF@rGO was obtained through the GO confinement effect and the cation-exchange reaction of purified inorganic–organic ZnS-ethylenediamine (ZnS(EN)0.5) NSs with Cd2+. The reaction between ethylenediamine (EN) in ZnS(EN)0.5 NS and GO sheet [18], and cation-exchange are the key roles for the formation of ZnxCd1−xS-NF@rGO with adjustable composition. Moreover, the as-prepared ZnxCd1−xS-NF@rGO has excellent photoelectronic response and photocatalytic activity under visible-light illumination as a result of the unique structures and composition natures, showing potential applications in photocatalysis and nanodevices.
2. Experimental section
2.1. Synthesis of ZnS(EN)0.5 inorganic–organic hybrid precursor
ZnS(EN)0.5 NSs were prepared by the reported methods [19] with some modifications. Typically, 0.01 mol ZnCl2 and 0.02 mol thiourea were dissolved in 30 ml ethylenediamine. The autoclave was maintained at 180 °C for 24 h. The white precipitate was filtered, washed with distilled water, and then washed with absolute ethanol to remove the residues of the impurities. The bright white products were dried under vacuum at 60 °C for 5 h before further characterization.
2.2. Synthesis of ZnxCd1−xS-NF@rGO
ZnS(en)0.5 NSs (0.1 mmol) and CdCl2 (0.05 mmol) were ultrasounded in de-ionized water for half an hour, and GO was ultrasounded in de-ionized water for two hours, then these two solutions were mixed with each other. The weight ratio of ZnS(en)0.5 NSs to GO is 1:2. The mixture solution was then stirred for 15 min and transferred into a Teflon-linked stainless steel autoclave (50 ml capacity). The autoclave was sealed and maintained at 180 °C for 12 h. After cooling down to room temperature spontaneously, the products are rinsed with distilled water and ethanol, and dried at 60 °C under vacuum for 2 h. By controlling the amount of CdCl2, partially exchanged production such as Zn0.4Cd0.6S-NF@rGO and Zn0.9Cd0.1S-NF@rGO can be produced.
2.3. Structural characterizations
The product morphology and crystal structure was examined using field-emission scanning electron microscopy equipped with an energy dispersive x-ray (EDX) system (FESEM; Hitachi, S5500), transmission electron microscopy (TEM; JEOL, JEM-2011, 200 kV; FEI, Tecnai G2 20, 200 kV), x-ray photospectroscopy (XPS, Escalab 250, Al Kα), and a Raman spectrometer (Renishaw RM 2000) equipped with a 514 nm diode laser. Crystallographic information regarding the samples was collected using Rigaku D/Max-2500 x-ray powder diffractometer (XRD) Cu-Kα irradiation (λ = 1.5418 Å).
2.4. Photocurrent response measurements
The photoelectronic properties were measured by a CHI 660B electrochemical system (Shanghai, China) using a standard cell with a working electrode, a platinum-wire counter electrode and a standard calomel electrode (SCE) reference electrode. Na2SO4 (0.1 M) was used as the electrolyte solution. The working electrode was prepared as follows: a tested sample of 15 mg was mixed with 0.5 ml water to produce slurry, which was then dip coated on a 20 mm × 40 mm indium−tin oxide (ITO) glass electrode; the electrode was then exposed in air for 8 h and subsequently heated at 100 °C for 1 day to eliminate water. All the investigated working electrodes were of the same thickness. The visible irradiation was obtained from a 500 W Xe lamp (Institute for Electric Light Sources, Beijing) with a 420 nm cutoff filter. Potentials were given with reference to the SCE. The photocurrent responses as light on and off were measured at 0 V.
2.5. Photocatalytic degradation methyl orange (MO) activity
25 mg of the as-prepared photocatalyst was dispersed in 50 ml of a MO aqueous solution (0.05 mmol l−1) and stirred for 5 h in the dark to reach adsorption–desorption equilibrium at room temperature. The experiments were carried out under visible light irradiation supplied by a 500 W Xe lamp with a UV cut-off filter (420 nm). About 4 ml of the solution was obtained at 30 min intervals under visible light irradiation, was separated by centrifugation to remove the photocatalyst and the degradation results were monitored by UV-visible spectra (Hitachi U-3010 spectrophotometer).
3. Results and discussion
Inorganic–organic ZnS(EN)0.5 NSs and GO were synthesized by solvothermal synthesis [19] and the modified Hummer's method [20], respectively. The precursors were examined using FESEM and XRD techniques (figures 1(a), (b), figure S1 in the supplementary information). It can be clearly seen that most ZnS(EN)0.5 NSs existed in the form of rectangles and GO sheets were quite thin and flexible. The GO confinement effect and oriented cation exchange reaction were realized by heating an aqueous solution containing ZnS(EN)0.5 NSs, GO sheets and Cd2+ at 180 °C for 12 h, as described in figure 1(c).
Figure 1. TEM images of (a) ZnS(EN)0.5 NSs and (b) GO sheets. (c) Schematic image of the reaction process, where the yellow spheres and small red spheres stand for Cd and Zn ions, respectively; the chemical bonds mean various oxygen-containing functional groups (such as C=O, C–OH, –COOH, and C–O–C) of GO sheets.
Download figure:
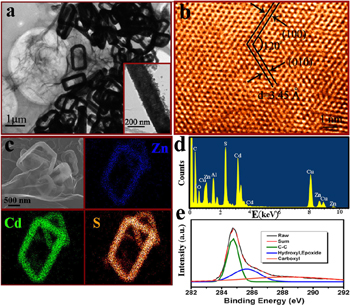
Standard image High-resolution imageUpon the reaction of the ZnS(EN)0.5 NSs with GO and Cd2+, the solid NSs evolved into hollow NFs. Figure 2(a) displays a typical TEM image of Zn0.75Cd0.25S-NF@rGO, of which the skeletons are about 150 nm (inset of figure 2(a)) consisting of nanoparticles of size ∼15 nm. The contrast of the graphene covering the NFs is invisible because it is so thin. High-resolution TEM image shows that the structure of the NFs can be determined as wurtzite type. The observed lattice space of the (100) plane of Zn0.75Cd0.25S is a little greater than that of ZnS, which may be due to the larger radius of Cd2+ than that of Zn2+ (figure 2(b)) [15]. The wrapping of graphene makes the scanning electron microscope (SEM) images of NFs a bit blurry (upper left corner of figure 2(c)). The element mappings (figure 2(c)) illustrate that the Zn, Cd and S elements are distributed uniformly in the NF skeletons, and the NF central region is almost absolutely hollow. The EDX result (figure 2(d)) shows that the products are composed of Zn, Cd, and S, and the atomic ratio is ∼3:1 for elements Zn to Cd, confirming the formation of Zn0.75Cd0.25S. The C1s peaks of the XPS spectra of the composites are outlined in figure 2(e). Compared to that of GO (figure S2 in the supplementary information), the intensity of peaks for the oxygen-containing functional group decreases obviously, which indicates the transformation of GO to rGO [21]. The reduction of GO is also verified by Raman spectra of the composites and GO sheets (figure S3 in the supplementary information).
Figure 2. (a) TEM image of Zn0.75Cd0.25S-NF@rGO. (b) High-resolution TEM image of a NF. (c) SEM image and elemental mappings of a typical NF. (d) EDS pattern of the composite. (e) C1s peak of the XPS spectrum of the composites.
Download figure:
Standard image High-resolution imageControl experiments were carried out to distinguish the effect of GO in the formation of NFs. Without the addtion of GO and other conditions kept consistent, porous NSs were obtained as shown in figure 3(b), in contrast to the NFs yielded by the addition of GO (figure 3(a)). When GO is replaced by g-C3N4, which has the similar morphology composed of flexible sheet (figure 3(c)), the outcome is also porous NSs (figure 3(d)). It showed clearly that GO played an important role in the formation of NFs.
Figure 3. Typical TEM images of the products obtained (a) with and (b) without addition of GO in the initial reaction system. (c) TEM images of g-C3N4. (d) TEM images of ZnS NS/g-C3N4. The insets are the close-ups of a single NF (a) and porous NS (b), (d).
Download figure:
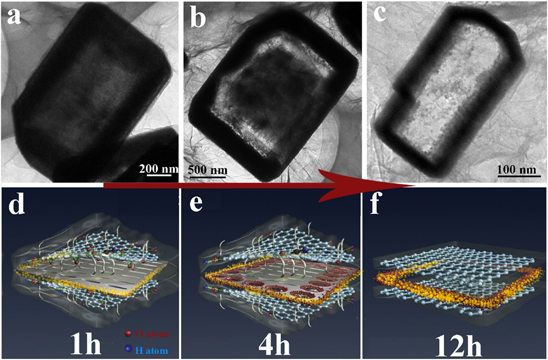
Standard image High-resolution imageTo have a deep insight into the generation of ZnxCd1−xS-NF@rGO, a series of time-dependent morphology evolution experiments were performed. Figure 4 depicts the morphology of the products obtained at different stages of the reaction. When the reaction was carried out for 1 h, the outline of the NSs arose and fine voids appeared within the NSs (figures 4(a), (d)). When the reaction was continued for 4 h, the sample contained large voids close to its side edges, but many nanoparticles (NPs) still existed in its center (figures 4(b), (e)). A prolonged reaction time to 12 h led to the complete transformation into Zn0.75Cd0.25S NF, and also the GO was reduced to rGO simultaneously (figures 4(c), (f)). Additional zoomed out TEM images for the products synthesized at different reaction times also support the above formation process of NFs from starting NSs (figure S4 in the supplementary information).
Figure 4. TEM and corresponding schematic images of products synthesized at different reaction times, 1 h (a), (d), 4 h (b), (e) and 12 h (c), (f). In the schematic images, one corner of the GO or rGO sheet is artificially lifted up to clearly exhibit the transformation process. The yellow spheres and small red spheres stand for Cd and Zn ions, respectively, the chemical bonds mean various oxygen-containing functional groups of GO sheets, the removing of the chemical bonds denotes that GO has been reduced to rGO.
Download figure:
Standard image High-resolution imageBased on the above results, we propose that the transformation mechanism of initial ZnS(en)0.5 inorganic–organic hybrid NSs into Zn0.75Cd0.25S NFs involves a GO confinement effect and an oriented cation exchange process. The thickness of the ZnS(en)0.5 NS is much smaller than its width and length, as displayed in figure 1(c). When the hybrid NSs are mixed with large surface-area GO sheets, the top/bottom surfaces of the NSs are wrapped tightly by GO sheets, while the side edges are exposed much more easily. Therefore, the GO-reduction reactions taking place on the EN in the NS side edges must be much less than those on the NS top/bottom surfaces. Thus, the severe consumption of EN occurs firstly on the top/bottom surface due to the interactions between EN molecules and oxygen-containing functional groups on the GO sheets, and drives the solid interior of the NS to break into discrete NPs. The NPs forming originally at the NS interior dissolve and then regrow on the side edges through the Ostwald ripening mechanism [9], leading to the appearance of many NPs on the side edges. The confinement also causes the NPs that have formed closer to the side edges to dissolve earlier than those farther away. Hence, large voids initially appear in the vicinity of the side edges and then expand towards the NS center. Finally, a hollow NF is obtained and the residual EN in its skeleton all reacts with residual oxygen-containing functional groups on the GO sheets, which are reduced to rGO sheets simultaneously. Meanwhile, the exposed side edges of the NS are more inclined to be exchanged by Cd2+. Here, the orientation of the cation exchange is expressed in that Cd2+ tends to attack the side edges preferentially instead of other surfaces in this specific environment.
During the formation of NFs, the confinement effect of GO is embodied in two parts. Firstly, it encloses the ZnS(en)0.5 NS in its interior, which determines different reaction environments between side edges and top/bottom surfaces. The top/bottom surfaces of the ZnS(en)0.5 NS are attached to the GO sheet and the side edges are almost suspended in the middle of the top/bottom GO sheets. This special structure makes the side edges easier to expose, which is of importance for oriented cation exchange. Secondly, the strong reaction driving force between EN and oxygen-containing functional groups makes EN disaffiliate quickly from the original NS, prompting the NS to split into nanoparticles. Therefore, NFs cannot be obtained by replacing GO with g-C3N4 nanosheets, since there is no strong interactions between EN molecules and g-C3N4 nanosheets which contain no oxygen-containing functional groups (figure 3(d)).
It should be pointed out here that the size of the obtained NFs is not uniform, which may be attributed to the broad size distribution of the ZnS(EN)0.5 inorganic–organic hybrid precursor. Compared to the ZnS(EN)0.5 hybrid NSs of large size, the value of the contact area between GO sheets and hybrid NSs is higher for small hybrid NSs. It is therefore believed that the transformation process from NSs to NFs should be size-dependent. Although it is difficult to synthesise an inorganic–organic hybrid precursor with a uniform size at present, the further in-depth study of the size effect of NF formation remains an interesting topic.
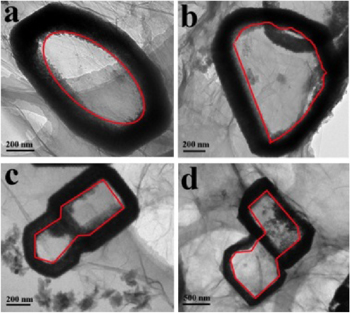
Except for the NFs with a rectangular shape, NFs with various shapes, which are determined by the initial shapes of the ZnS(en)0.5 precursor NSs (figure 5), can also be obtained. This close correspondence implies that NFs with diverse shapes can be realized and proves that our strategy with the synergistic actions of the GO confinement effect and oriented cation exchange is applicable to extensive hollow nanostructures.
Figure 5. TEM images of Zn0.75Cd0.25S NFs with various morphologies.
Download figure:
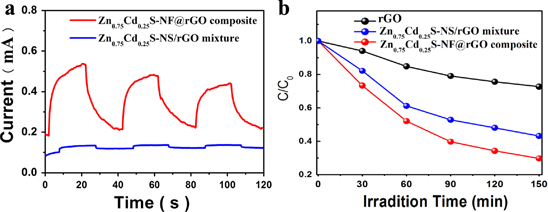
Standard image High-resolution imageRecently, ZnxCd1−xS has been demonstrated as a high-performance catalyst for efficient hydrogen-evolution reactions [22, 23]. The utilization of the high surface area and electrical conductance of graphene can lead to enhanced photocatalytic performance [24]. Our strategy can be used to synthesise ZnxCd1−xS-NF@rGO with different atomic ratios of Zn: Cd by simply changing the mass of the precursors. TEM and SEM images show that NFs with various compositions can be obtained (figure S5 in the supplementary information). XRD patterns show that the diffraction peaks of ZnxCd1−xS approach a small angle with the increase in Cd content (figure S6 in the supplementary information), which is due to the Cd2+ with a larger radius (0.97 Å and 0.74 Å for Cd2+ and Zn2+) incorporated in the ZnS lattice entering into lattice and/or interstitial sites. The doped Cd element decreases the bandgap and shifts the absorption edge of ZnS into the visible light range [25]. Under visible irradiation, electrons are excited from the valence band (VB) composed of S 3p, to the CB which is hybridized by Zn 4s4p and Cd 5s5p [15]. Figure 6(a) displays the photocurrent responses of the Zn0.75Cd0.25S-NF@rGO composite and the mechanical mixture by Zn0.75Cd0.25S-NS (figure 3(b)) and rGO (Zn0.75Cd0.25S-NS/rGO) during repeated on/off cycles of visible light. The photocurrent of Zn0.75Cd0.25S-NF@rGO is sensitive and steady. The short-circuit photocurrent density is 0.3 mA, which is seven times that of Zn0.75Cd0.25S-NS/rGO. In figure 6(b), we can see that the Zn0.75Cd0.25S-NF@rGO displays excellent photocatalytic activities. The MO dye can be decomposed by 70% within 150 min of visible light irradiation, while the Zn0.75Cd0.25S-NS/rGO and rGO display much less activity. The enhanced photoelectronic response and photocatalytic activity originate from the formation of NFs in situ on rGO and the close contact between NF and rGO, making it easier for electrons to transfer to graphene, which can effectively improve the charge separation. In addition, the hollow NF structure will expose many more active sites and decrease the agglomeration of nanoparticles. Above all, the as-prepared Zn0.75Cd0.25S-NF@rGO shows promise for applications in photoeletronic and photocatalytic fields.
Figure 6. (a) Transient photocurrent responses of Zn0.25Cd0.75S-NF@rGO and Zn0.25Cd0.75S-NS/rGO with visible light on/off cycles. (b) The photodecomposition curve of MO. C0 is the original concentration of MO after the adsorption–desorption equilibrium and C is the concentration of the remaining MO at time t.
Download figure:
Standard image High-resolution image4. Conclusions
In conclusion, a novel strategy for the synthesis of multicomponent NF structure via the synergistic actions of the GO confinement effect and oriented cation exchange has been proposed. The thin GO sheets which enclose the NS determine different reaction environments in side edges and top/bottom surfaces. The severe consumption of EN by the oxygen-containing functional group of GO in the central part and oriented cation exchange reaction on the edge sides both promote the formation of the multicomponent NFs. The high photoelectrochemical and photocatalytic activity, and the low cost of the starting materials, will make it applicable in photoelectronic and photocatalytic fields. Further hydrogen evolution reaction research based on these special structures are under way.
Acknowledgments
The authors appreciate the financial support from the Chinese National Natural Scientific Foundation (51571054, 51401114). This work made use of the resources of the Beijing National Center for Electron Microscopy.
Supplementary information
XRD pattern of the as-prepared hybrid ZnS(EN)0.5 NSs, high-resolution of C1s spectrum of the as-prepared GO, Raman spectra of GO and Zn0.75Cd0.25S-NF@rGO, TEM and SEM images of ZnxCd1−xS-NF@rGO, XRD patterns of the as-prepared ZnxCd1−xS-NF@rGO.