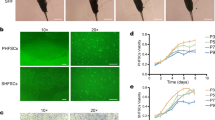
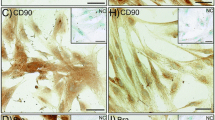
Abstract
Fetal-maternal microchimerism describes the acquisition of fetal stem cells (FSC) by the mother during pregnancy and their long-term persistence after parturition. FSC may engraft in a variety of maternal tissues especially if there is organ/tissue injury, but their role and mechanism of persistence still remains elusive. Clinical applications due to their pluripotency, immunomodulatory effects and accessibility make them good candidates for ex-vivo manipulation and autologous therapies. The hair follicles contain a distinctive niche for pluripotent stem cells (PSC). To date, there is no published evidence of fetal microchimerism in the hair follicle. In our study, follicular unit extraction (FUE) technique allowed easy stem cell cultures to be obtained while simple hair follicle removal by pull-out technique failed to generate stem cells in culture. We identified microchimeric fetal stem cells within the primitive population of maternal stem cells isolated from the hair follicles with typical mesenchymal phenotype, expression of PSC genes and differentiation potential towards osteocytes, adypocites and chondrocytes. This is the first study to isolate fetal microchimeric stem cells in adult human hair long after parturition. We presume a sanctuary partition mechanism with PSC of the mother deposited during early embryogenesis could explain their long-term persistence.






Similar content being viewed by others
References
Levy, V., Lindon, C., Harfe, B. D., & Morgan, B. A. (2005). Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Developmental Cell, 9, 855–861. https://doi.org/10.1016/j.devcel.2005.11.003.
Hong, Y. C., Liu, H. M., Chen, P. S., et al. (2007). Hair follicle: a reliable source of recipient origin after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplantation, 40(9), 871–874.
Yu, H., Fang, D., Kumar, S. M., et al. (2006). Isolation of a novel population of multipotent adult stem cells from human hair follicles. The American Journal of Pathology, 168, 1879–1888. https://doi.org/10.2353/ajpath.2006.051170.
Boddy, A. M., Fortunato, A., Wilson Sayres, M., & Aktipis, A. (2015). Fetal microchimerism and maternal health: a review and evolutionary analysis of cooperation and conflict beyond the womb. Bioessays, 37(10), 1106–1118.
Magued, M., Hamdi, H., & Welsh, J. (2008). High frequency of fetal cells within a primitive stem cell population in maternal blood. Human Reproduction, 23(4), 928–933.
Ratajczak, M., Machalinski, B., Wojakowski, W., Ratajczak, J., & Kucia, M. (2007). A hypothesis for an embryonic origin of pluripotent Oct-4 + stem cells in adult bone marrow and other tissues. Leukemia, 21, 860–867.
Cismaru, C. A., Pop, L., & Berindan-Neagoe, I. (2018). Incognito: Are Microchimeric Fetal Stem Cells that Cross Placental Barrier Real Emissaries of Peace? Stem Cell Reviews, 2018. https://doi.org/10.1007/s12015-018-9834-9.
Shimazaki, C., Ochiai, N., Uchida, R., et al. (2002). Non-T cell-depleted HLA haploidentical stem cell transplantation in advanced hematologic malignancies based on the feto-maternal michrochimerism. Blood, 101(8), 3334–3336.
Fugazzola, L., Cirello, V., & Beck-Peccoz, P. (2011). Fetal microchimerism as an explanation of disease. Nature Reviews Endocrinology, 7, 89–97.
Kucia, M., Reca, R., Campbell, F. R., et al. (2006). A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia, 20, 857–869.
Kucia, M., Halasa, M., Wysoczynski, M., et al. (2007). Morphological and molecular characterization of novel population of CXCR4 + SSEA-4 + Oct-4+ very small embryonic-like (VSEL) cells purified from human cord blood – preliminary report. Leukemia, 21, 297–303.
Kruse, C., Kajahn, J., Petschnik, A. E., et al. (2006). Adult pancreatic stem/progenitor cells spontaneously differentiate in vitro into multiple cell lineages and form teratoma-like structures. Annals of Anatomy, 188, 503–517.
Guan, K., Nayernia, K., Maier, L. S., et al. (2006). Pluripotency of spermatogonial stem cells from adult mouse testis. Nature, 440, 1199–1203.
Ling, T. Y., Kuo, M. D., Li, C. L., et al. (2006). Identification of pulmonary Oct-4+ stem/progenitor cells and demonstration of their susceptibility to SARS coronavirus (SARS-CoV) infection in vitro. Proceedings of the National Academy of Sciences of the United States of America, 103, 9530–9535.
Okita, K., Ichisaka, T., & Yamanaka, S. (2007). Generation of germline-competent induced pluripotent stem cells. Nature, 448(7151), 313–317.
Kim, J. B., Sebastiano, V., Wu, G., et al. (2009). Oct4-induced pluripotency in adult neural stem cells. Cell., 136(3), 411–419.
Mihu, C. M., Rus Ciuca, D., Soritau, O., Susman, S., & Mihu, D. (2009). Isolation and characterization of mesenchymal stem cells from the amniotic membrane. Romanian Journal of Morphology and Embryology, 50(1), 73–77.
Pochampally, R. R., Smith, J. R., Ylostalo, J., & Prockop, D. J. (2004). Serum deprivation of human marrow stromal cells (hMSCs) selects for a subpopulation of early progenitor cells with enhanced expression of OCT-4 and other embryonic genes. Blood, 103, 1647–1652.
Jiang, Y., Jahagirdar, B. N., Reinhardt, R. L., et al. (2002). Pluripotency of mesenchymal stem cells derived from adult marrow. Nature, 418, 41–49.
D’Ippolito, G., Diabira, S., Howard, G. A., Menei, P., Roos, B. A., & Schiller, P. C. (2004). Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. Journal of Cell Science, 117, 2971–2981.
Kogler, G., Sensken, S., Airey, J. A., et al. (2004). A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. The Journal of Experimental Medicine, 200, 123–135.
Nayernia, K., Lee, J. H., Drusenheimer, N., et al. (2006). Derivation of male germ cells from bone marrow stem cells. Laboratory Investigation, 86, 654–663.
Johnson, J., Bagley, J., Skaznik-Wikiel, M., et al. (2005). Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell, 122, 303–315.
Maloney, S., Smith, A., Furst, D. E., et al. (1999). Microchimerism of maternal origin persists into adult life. The Journal of Clinical Investigation, 104, 41–47.
Lo, Y. M., Lo, E. S., Watson, N., Noakes, L., Sargent, I. L., Thilaganathan, B., & Wainscoat, J. S. (1996). Twoway cell traffic between mother and fetus: biologic and clinical implications. Blood, 88, 4390–4395.
Virchow, R. (1855). Editorial Archive fuer pathologische. Anatomie und Physiologie fuer klinische Medizin, 8, 23–54.
Rovó, A., Meyer-Monard, S., Heim, D., et al. (2005). No evidence of plasticity in hair follicles of recipients after allogeneic hematopoietic stem cell transplantation. Experimental Hematology, 33(8), 909–911.
Santurtún, A., Riancho, J. A., Santurtún, M., et al. (2017). Genetic DNA profile in urine and hair follicles from patients who have undergone allogeneic hematopoietic stem cell transplantation. Science & Justice, 57(5), 336–340.
Li, Y. T., Xie, M. K., & Wu, J. (2014). DNA profiling in peripheral blood, buccal swabs, hair follicles and semen from a patient following allogeneic hematopoietic stem cells transplantation. Biomed Rep, 2(6), 804–808.
Kaiser, S., Hackanson, B., Follo, M., et al. (2007). BM cells giving rise to MSC in culture have heterogeneous CD34 and CD45 phenotype. Cytotherapy, 9, 439–450.
Jacewicz, R., Lewandowski, K., Rupa-Matysek, J., et al. (2010). Donor-derived DNA in hair follicles of recipients after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplantation, 45(11), 1638–1644.
Ventura Ferreira, M. S., Bienert, M., Müller, K., et al. (2018). Comprehensive characterization of chorionic villi-derived mesenchymal stromal cells from human placenta. Stem Cell Research & Therapy, 9, 28.
Jay Iams, Creasy, Robert K., Resnik, Robert, Resnik, Robert (2004). Maternal-fetal medicine. Philadelphia: W.B. Saunders Co. pp. 31–32. ISBN 0-7216-0004-2.
Lash, G., Robson, S., & Bulmer, J. (2010). Review: Functional role of uterine natural killer (uNK) cells in human early pregnancy decidua. Placenta., 31(S), 87–92. https://doi.org/10.1016/j.placenta.2009.12.022.
Lamar, D. L., Weyand, C. M., & Goronzy, J. J. (2010). Promoter choice and translational repression determine cell type–specific cell surface density of the inhibitory receptor CD85j expressed on different hematopoietic lineages. Blood., 115(16), 3278–3286.
Colonna, M., Samaridis, J., Cella, M., et al. (1998). Cutting Edge: Human Myelomonocytic Cells Express an Inhibitory Receptor for Classical and Nonclassical MHC Class I Molecules. Journal of Immunology, 160, 3096–3100.
Steven, A., & Seliger, B. (2018). The Role of Immune Escape and Immune Cell Infiltration in Breast Cancer. Breast Care (Basel), 13(1), 16–21.
Di Cristofaro, J., Karlmark, K. R., Kanaan, S. B., et al. (2018). Soluble HLA-G Expression Inversely Correlates With Fetal Microchimerism Levels in Peripheral Blood From Women With Scleroderma. Frontiers in Immunology, 9, 1685.
Cirello, V., Recalcati, M. P., Muzza, M., et al. (2008). Fetal cell microchimerism in papillary thyroid cancer: a possible role in tumor damage and tissue repair. Cancer Research, 15, 8482–8488.
Gyurkocza, B., & Sandmaier, B. M. (2014). Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood., 124(3), 344–353.
Cell Trialists’ Collaborative Group. (2005). Allogeneic peripheral blood stem-cell compared with bone marrow transplantation in the management of hematologic malignancies: an individual patient data meta-analysis of nine randomized trials. Journal of Clinical Oncology, 23(22), 5074–5087.
Zhang, H., Chen, J., & Que, W. (2012). Allogeneic peripheral blood stem cell and bone marrow transplantation for hematologic malignancies: meta-analysis of randomized controlled trials. Leukemia Research, 36(4), 431–437.
Okada, M., Yoshihara, S., Taniguchi, K., et al. (2012). Intrabone Marrow Transplantation of Unwashed Cord Blood Using Reduced-Intensity Conditioning Treatment: A Phase I Study. Biology of Blood and Marrow Transplantation, 18, 633–639.
Toprak, S. K. (2018). Donor lymphocyte infusion in myeloid disorders. Transfusion and Apheresis Science, 57(2), 178–186.
Krampera, M., Glennie, S., Dyson, J., et al. (2003). Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood, 101(9), 3722–3729.
Yu, J., Ren, X., Cao, S., Li, H., & Hao, X. (2008). Beneficial effects of fetal-maternal microchimerism on the activated haplo-identical peripheral blood stem cell treatment for cancer. Cytotherapy, 10(4), 331–339.
Ying H, Jinpu Y, Shui C, et al. (2010). Fetal–Maternal Microchimerism Enhances the Survival Effect of Interleukin-2-Activated Haploidentical Peripheral Blood Stem Cell Treatment in Patients with Advanced Solid Cancer. Cancer Biotherapy and Radiopharmaceuticals, 25(6, Mary Ann Liebert, Inc.).
Corcione, A., Benvenuto, F., Ferretti, E., et al. (2006). Human mesenchymal stem cells modulate B cell functions. Blood, 2006(107), 367–372. https://doi.org/10.1182/blood-2005-07-2657.
Wang, L., Zhu, C., Ma, D., et al. (2018). Efficacy and safety of mesenchymal stromal cells for the prophylaxis of chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation: a meta-analysis of randomized controlled trials. Annals of Hematology, 97, 1941.
Acknowledgements
Part of the research was supported from the grant 108BG 01/10/2016) PN-III-P2-2.1-BG-2016-0117 directed by Assoc. prof. Sergiu Susman. The the work was done at the Research Center for Functional Genomics, Biomedicine and Translational Medicine of the University of Medicine and Pharmacy Iuliu Hatieganu.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
The study was approved by the ethics committee of the University of Medicine and Pharmacy “Iuliu Hatieganu” Cluj-Napoca, reg. no. 100/08.03.2017.
Conflict of Interests
All authors have read and approved this version of the article, and due care has been taken to ensure the integrity of the work. No part of this article has been published or submitted elsewhere. No financial conflict of interest exists in the submission of this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cismaru, C.A., Soritau, O., Jurj, A . et al. Isolation and Characterization of a Fetal-Maternal Microchimeric Stem Cell Population in Maternal Hair Follicles Long after Parturition. Stem Cell Rev and Rep 15, 519–529 (2019). https://doi.org/10.1007/s12015-019-09885-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-019-09885-4