Abstract
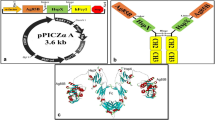
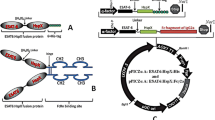
As an ancient disease, tuberculosis (TB) is a major global health threat. Therefore, there is an urgent need for an effective and safe anti-TB vaccine. In the current study, a delivery system of Fc domain of mouse IgG2a and early secreted antigenic target protein 6 (ESAT-6) was evaluated for the selective uptake of antigens by antigen-presenting cells (APCs). Thus, it was based on the immunogenicity of a fusion protein. The study was initiated by the transfer of recombinant expression vectors of pPICZαA-ESAT-6:Fcγ2a and pPICZαA-ESAT-6: His into Pichia pastoris (P. pastoris). Recombinant proteins were assessed for immunogenicity following the immunoblotting analysis. High levels of IFN-γ and IL-12 were produced to induce Th1-type cellular responses through vaccination with both recombinant proteins [ESAT-6:Fcγ2a (EF) and ESAT-6:His (EH)]. The Fc-tagged recombinant protein induced more effective Th1-type cellular responses with a low increment in IL-4 compared to PBS, BCG, and EH groups. Although in all the immunized groups, the ratio of IFN-γ/IL-4 was in favor of Th1 responses, the highest Th1/Th2 balance was observed in EF immunized group. Fc fragment of mouse IgG2a may induce a selective uptake of APCs towards the cross-presentation and formation of Th1 responses in favor of an appropriate protective anti-tuberculosis reaction. Thus, further research on Fc-fusion proteins is required to develop Fc-based TB vaccines.





Similar content being viewed by others
References
World Health Organization (2015) Global tuberculosis report 2015 (WHO). www.who.int/about/licensing/copyright_form/en/index.html
Nuttall JJ, Davies MA, Hussey GD, Eley BS (2008) Bacillus Calmette-Guerin (BCG) vaccine-induced complications in children treated with highly active antiretroviral therapy. Int J Infect Dis 12:e99–e105
Logan KE, Chambers MA, Hewinson RG, Hogarth PJ (2005) Frequency of IFN-gamma producing cells correlates with adjuvant enhancement of bacille Calmette-Guerin induced protection against Mycobacterium bovis. Vaccine 23:5526–5532
Behr MA, Wilson MA, Gill WP, Salamon H, Schoolnik GK, Rane S et al (1999) Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 284:1520–1523
Brodin P, Rosenkrands I, Andersen P, Cole ST, Brosch R (2004) ESAT-6 proteins: protective antigens and virulence factors? Trends Microbiol 12:500–508
Jiang Q, Zhang J, Chen X, Xia M, Lu Y, Qiu W et al (2013) A novel recombinant DNA vaccine encoding Mycobacterium tuberculosis ESAT-6 and FL protects against Mycobacterium tuberculosis challenge in mice. J Biomed Res 27:406–420
Brandt L, Elhay M, Rosenkrands I, Lindblad EB, Andersen P (2000) ESAT-6 subunit vaccination against Mycobacterium tuberculosis. Infect Immun 68:791–795
Weinrich Olsen A, van Pinxteren LA, Meng Okkels L, Birk Rasmussen P, Andersen P (2001) Protection of mice with a tuberculosis subunit vaccine based on a fusion protein of antigen 85b and esat-6. Infect Immun 69:2773–2778
Zaghouani H, Steinman R, Nonacs R, Shah H, Gerhard W, Bona C (1993) Presentation of a viral T cell epitope expressed in the CDR3 region of a self immunoglobulin molecule. Science 259:224–227
Carter PJ (2011) Introduction to current and future protein therapeutics: a protein engineering perspective. Exp Cell Res 317:1261–1269
Adamova E, Walsh MC, Gosselin DR, Hale K, Preissler MT, Graziano RF et al (2005) Enhanced antigen-specific antibody and cytokine responses when targeting antigen to human Fc-gamma receptor type I using an anti-human Fc-gamma receptor type I-streptavidin fusion protein in an adjuvant-free system. Immunol Invest 34:417–429
Keler T, Guyre PM, Vitale LA, Sundarapandiyan K, van De Winkel JG, Deo YM et al (2000) Targeting weak antigens to CD64 elicits potent humoral responses in human CD64 transgenic mice. J Immunol 165:6738–6742
Rawool DB, Bitsaktsis C, Li Y, Gosselin DR, Lin Y, Kurkure NV et al (2008) Utilization of Fc receptors as a mucosal vaccine strategy against an intracellular bacterium, Francisella tularensis. J Immunol 180:5548–5557
Walsh MC, Banas JA, Mudzinski SP, Preissler MT, Graziano RF, Gosselin EJ (2003) A two-component modular approach for enhancing T-cell activation utilizing a unique anti-FcgammaRI-streptavidin construct and microspheres coated with biotinylated-antigen. Biomol Eng 20:21–33
Soleimanpour S, Farsiani F, Mosavat A, Ghazvini K, Eydgahi MR, Sankian M et al (2015) APC targeting enhances immunigenicity of a novel multistage Fc-fusion tuberculosis vaccine in mice. Appl Microbiol Biotechnol 99:10467–10480
den Haan JM, Lehar SM, Bevan MJ (2000) CD8(+) but not CD8(−) dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med 192:1685–1696
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Davidsen J, Rosenkrands I, Christensen D, Vangala A, Kirby D, Perrie Y et al (2005) Characterization of cationic liposomes based on dimethyldioctadecylammonium and synthetic cord factor from M. tuberculosis (trehalose 6,6′-dibehenate)-a novel adjuvant inducing both strong CMI and antibody responses. Biochim Biophys Acta 1718:22–31
Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR (1993) An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med 178:2249–2254
Lockhart E, Green AM, Flynn JL (2006) IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol 177:4662–4669
Cooper AM (2009) Cell-mediated immune responses in tuberculosis. Annu Rev Immunol 27:393–422
Mosmann TR, Coffman RL (1989) TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 7:145–173
Ansel KM, Lee DU, Rao A (2003) An epigenetic view of helper T cell differentiation. Nat Immunol 4:616–623
Wang X, Barnes PF, Huang F, Alvarez IB, Neuenschwander PF, Sherman DR et al (2012) Early secreted antigenic target of 6-kDa protein of Mycobacterium tuberculosis primes dendritic cells to stimulate Th17 and inhibit Th1 immune responses. J Immunol 189:3092–3103
Wang X, Barnes PF, Dobos-Elder KM, Townsend JC, Chung YT, Shams H et al (2009) ESAT-6 inhibits production of IFN-gamma by Mycobacterium tuberculosis-responsive human T cells. J Immunol 182:3668–3677
Pathak SK, Basu S, Basu KK, Banerjee A, Pathak S, Bhattacharyya A et al (2007) Direct extracellular interaction between the early secreted antigen ESAT-6 of Mycobacterium tuberculosis and TLR2 inhibits TLR signaling in macrophages. Nat Immunol 8:610–618
Langermans JA, Doherty TM, Vervenne RA, van der Laan T, Lyashchenko K, Greenwald R et al (2005) Protection of macaques against Mycobacterium tuberculosis infection by a subunit vaccine based on a fusion protein of antigen 85B and ESAT-6. Vaccine 23:2740–2750
Pym AS, Brodin P, Majlessi L, Brosch R, Demangel C, Williams A et al (2003) Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat Med 9:533–539
Olsen AW, Williams A, Okkels LM, Hatch G, Andersen P (2004) Protective effect of a tuberculosis subunit vaccine based on a fusion of antigen 85B and ESAT-6 in the aerosol guinea pig model. Infect Immun 72:6148–6150
Sereinig S, Stukova M, Zabolotnyh N, Ferko B, Kittel C, Romanova J et al (2006) Influenza virus NS vectors expressing the mycobacterium tuberculosis ESAT-6 protein induce CD4 + Th1 immune response and protect animals against tuberculosis challenge. Clin Vaccine Immunol 13:898–904
Agger EM, Rosenkrands I, Hansen J, Brahimi K, Vandahl BS, Aagaard C et al (2008) Cationic liposomes formulated with synthetic mycobacterial cordfactor (CAF01): a versatile adjuvant for vaccines with different immunological requirements. PLoS One 3:e3116
Korepanova A, Gao FP, Hua Y, Qin H, Nakamoto RK, Cross TA (2005) Cloning and expression of multiple integral membrane proteins from Mycobacterium tuberculosis in Escherichia coli. Protein Sci 14:148–158
Andersson GE, Sharp PM (1996) Codon usage in the Mycobacterium tuberculosis complex. Microbiology 142:915–925
Li P, Anumanthan A, Gao XG, Ilangovan K, Suzara VV, Duzgunes N et al (2007) Expression of recombinant proteins in Pichia pastoris. Appl Biochem Biotechnol 142:105–124
Ordway DJ, Costa L, Martins M, Silveira H, Amaral L, Arroz MJ et al (2004) Increased Interleukin-4 production by CD8 and gammadelta T cells in health-care workers is associated with the subsequent development of active tuberculosis. J Infect Dis 190:756–766
Demissie A, Abebe M, Aseffa A, Rook G, Fletcher H, Zumla A et al (2004) Healthy individuals that control a latent infection with Mycobacterium tuberculosis express high levels of Th1 cytokines and the IL-4 antagonist IL-4delta2. J Immunol 172:6938–6943
Harris J, De Haro SA, Master SS, Keane J, Roberts EA, Delgado M et al (2007) T helper 2 cytokines inhibit autophagic control of intracellular Mycobacterium tuberculosis. Immunity 27:505–517
Reljic R, Paul MJ, Arias MA (2009) Cytokine therapy of tuberculosis at the crossroads. Expert Rev Respir Med 3:53–66
Goonetilleke NP, McShane H, Hannan CM, Anderson RJ, Brookes RH, Hill AV (2003) Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of bacille Calmette-Guerin vaccine using mucosal administration and boosting with a recombinant modified vaccinia virus Ankara. J Immunol 171:1602–1609
Heyman B (2000) Regulation of antibody responses via antibodies, complement, and Fc receptors. Annu Rev Immunol 18:709–737
Probst HC, Lagnel J, Kollias G, van den Broek M (2003) Inducible transgenic mice reveal resting dendritic cells as potent inducers of CD8 + T cell tolerance. Immunity 18:713–720
Goldenberg MM (1999) Etanercept, a novel drug for the treatment of patients with severe, active rheumatoid arthritis. Clin Ther 21:75–87
Farsiani H, Mosavat A, Soleimanpour S, Sadeghian H, Akbar Eydgahi MR, Ghazvini K, Sankian M et al (2016) Fc-based delivery system enhances immunogenicity of a tuberculosis subunit vaccine candidate consisting of the ESAT-6:CFP-10 complex. Mole Biosyst. doi:10.1039/c6mb00174b
Soleimanpour S, Hassannia T, Motiee M, Amini AA, Rezaee SAR (2016) Fcγ1 fragment of IgG1 as a powerful affinity tag in recombinant Fc-fusion proteins: immunological, biochemical and therapeutic properties. Crit Rev Biotechnol 6:1–22
Acknowledgments
This study has been supported by vice chancellor for research of Mashhad University of Medical Sciences (Grant no. 910072). This study was a part of a PhD dissertation by Abdollah Kebriaei.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Kebriaei, A., Derakhshan, M., Meshkat, Z. et al. Construction and immunogenicity of a new Fc-based subunit vaccine candidate against Mycobacterium tuberculosis . Mol Biol Rep 43, 911–922 (2016). https://doi.org/10.1007/s11033-016-4024-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-016-4024-9