Abstract
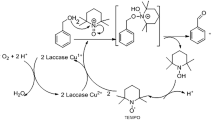
This work investigated the transformation of triclosan (TCS) by the laccase produced by a pathogen isolated from rotten tomato. The pathogen was characterized as Botrytis sp. FQ, belonging to subphylum Deuteromycotina. The laccase exhibited cold-adaptation with relatively high activity at 20°C. The laccase could effectively transform TCS. Approximately 62% TCS could be removed at dose of 1.0 unit∙mL–1 in 120 min. The reaction rate appeared to be pseudo-first-order to the concentration of the substrate, suggesting the laccase activity remained stable during the reaction. Transformation products of TCS were analyzed by mass spectrometry and it was revealed that TCS dimers were formed via radical coupling pathways. During this process, laccase catalyzed oxidation of TCS to form a radical intermediate is the rate limiting step. However, this step can be reversed by humic acid. Overall, the laccase showed great potential in the treatment of phenolic contaminants. Since laccase is widely presented in natural environment, this study also revealed an important pathway involved in the transformation of phenolic contaminants in the environment.

Similar content being viewed by others
References
Gujjala L K S, Bandyopadhyay T K, Banerjee R. Kinetic modelling of laccase mediated delignification of Lantana camara. Bioresource Technology, 2016, 212: 47–54
Nguyen L N, Hai F I, Dosseto A, Richardson C, PriceW E, Nghiem L D. Continuous adsorption and biotransformation of micropollutants by granular activated carbon-bound laccase in a packed-bed enzyme reactor. Bioresource Technology, 2016, 210: 108–116
Mao L, Huang Q, Luo Q, Lu J, Yang X, Gao S. Ligninase-mediated removal of 17ß-estradiol from water in the presence of natural organic matter: efficiency and pathways. Chemosphere, 2010, 80(4): 469–473
Mao L, Lu J, Habteselaßsie M, Luo Q, Gao S, Cabrera M, Huang Q. Ligninase-mediated removal of natural and synthetic estrogens from water: II. Reactions of 17ß-estradiol. Environmental Science & Technology, 2010, 44(7): 2599–2604
Litthauer D, Van Vuuren M J, Van Tonder A, Wolfaardt F W. Purification and kinetics of a thermostable laccase from Pycnoporus sanguineus (SCC 108). Enzyme and Microbial Technology, 2007, 40(4): 563568
Liu J Y, Cai Y J, Liao X R, Huang Q G, Hao Z H, HuMM, Zhang D B, Li Z L. Efficiency of laccase production in a 65-liter air-lift reactor for potential green industrial and environmental application. Journal of Cleaner Production, 2013, 39(1): 154160
Wang Z X, Cai Y J, Liao X R, Tao G J, Li Y Y, Zhang F, Zhang D B. Purification and characterization of two thermostable laccases with high cold adapted characteristics from Pycnoporus sp.SYBC-L1. Process Biochemistry, 2010, 45(10): 17201729
Buth J M, Steen P O, Sueper C, Blumentritt D, Vikesland P J, Arnold W A, McNeill K. Dioxin photoproducts of triclosan and its chlorinated derivatives in sediment cores. Environmental Science & Technology, 2010, 44(12): 4545–4551
Halden R U. On the need and speed of regulating triclosan and triclocarban in the United States. Environmental Science & Technology, 2014, 48(7): 3603–3611
Dann A B, Hontela A. Triclosan: environmental exposure, toxicity and mechanisms of action. Journal of Applied Toxicology, 2011, 31(4): 285–311
Fiss E M, Rule K L, Vikesland P J. Formation of chloroform and other chlorinated byproducts by chlorination of triclosan-containing antibacterial products. Environmental Science & Technology, 2007, 41(7): 2387–2394
Fiss E M, Rule K L, Vikesland P J. Formation of chloroform and other chlorinated byproducts by chlorination of triclosan-containing products. Environmental Science & Technology, 2008, 42(3): 976976
Latch D E, Packer J L, Stender B L, VanOverbeke J, Arnold W A, McNeill K. Aqueous photochemistry of triclosan: formation of 2,4- dichlorophenol, 2,8-dichlorodibenzo-p-dioxin, and oligomerization products. Environmental Toxicology and Chemistry, 2005, 24(3): 517–525
Zhang H, Huang C H. Oxidative transformation of triclosan and chlorophene by manganese oxides. Environmental Science & Technology, 2003, 37(11): 2421–2430
Li J, Peng J, Zhang Y, Ji Y, Shi H, Mao L, Gao S. Removal of triclosan via peroxidases-mediated reactions in water: reaction kinetics, products and detoxification. Journal of Hazardous Materials, 2016, 310: 152–160
Diwaniyan S, Kharb D, Raghukumar C, Kuhad R. Decolorization of synthetic dyes and textile effluents by basidiomycetous fungi. Water, Air, and Soil Pollution, 2010, 210(1–4): 409419
Tien M, Kirk T K. Lignin peroxidase of Phanerochaete chrysosporium. Methods in Enzymology, 1988, 161: 238249
Camarero S, Ibarra D, Martínez M J, Martínez A T. Lignin-derived compounds as efficient laccase mediators for decolorization of different types of recalcitrant dyes. Applied and Environmental Microbiology, 2005, 71(4): 1775–1784
Yu H, Sutton J C. Morphological development and interactions of Gliocladium roseum and Botrytis cinerea in raspberry. Canadian Journal of Plant Pathology-Revue Canadienne De Phytopathologie, 1997, 19(3): 237246
Morgan W M. The effect of night temperature and glasshouse ventilation on the incidence of Botrytis cinerea in a late-planted tomato crop. Crop Protection (Guildford, Surrey), 1984, 3(2): 243251
Swadling I R, Jeffries P. Antagonistic properties of two bacterial biocontrol agents of grey mould disease. Biocontrol Science and Technology, 1998, 8(3): 439448
Amerine MA. The search for good wine. Science, 1966, 154(3757): 1621–1628
Zouari N, Romette J L, Thomas D. Purification and properties of two laccase isoenzymes produced by Botrytis cinerea. Applied Biochemistry and Biotechnology, 1987, 15(3): 213225
Baldrian P. Fungal laccases—Occurrence and properties. FEMS Microbiology Reviews, 2006, 30(2): 215–242
Kim Y, Yeo S, Kim M K, Choi H T. Removal of estrogenic activity from endocrine-disrupting chemicals by purified laccase of Phlebia tremellosa. FEMS Microbiology Letters, 2008, 284(2): 172–175
Wang Z, Cai Y, Liao X, Zhang F, Zhang D, Li Z. Production and characterization of a novel laccase with cold adaptation and high thermal stability from an isolated fungus. Applied Biochemistry and Biotechnology, 2010, 162(1): 280–294 doi:10.1007/s12010-009-8801-y
Auriol M, Filali-Meknassi Y, Adams C D, Tyagi R D. Natural and synthetic hormone removal using the horseradish peroxidase enzyme: temperature and pH effects. Water Research, 2006, 40(15): 2847–2856
Lu J, Huang Q, Mao L. Removal of acetaminophen using enzymemediated oxidative coupling processes: I. Reaction rates and pathways. Environmental Science & Technology, 2009, 43(18): 7062–7067
Murray C A, Parsons S A. Removal of NOM from drinking water: Fenton’s and photo-Fenton’s processes. Chemosphere, 2004, 54(7): 1017–1023
Acknowledgments
The work was supported by the National Natural Science Foundation of China (Grant No. 51503074), the Fundamental Research Funds for Central Universities (KYZ201626) and the priority Academic Program Development (PAPD) of Jiangsu Higher Education Institute. We would like to thank Kang Wu (SEM laboratory, Jiangnan University, Wuxi, China) for his assistance in SEM observation.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Shi, Y., Kong, D., Liu, J. et al. Transformation of triclosan by a novel cold-adapted laccase from Botrytis sp. FQ. Front. Environ. Sci. Eng. 11, 6 (2017). https://doi.org/10.1007/s11783-017-0927-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11783-017-0927-5