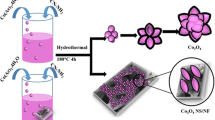
Abstract
It is highly attractive but still remains a great challenge to develop an efficient electrocatalyst for oxygen evolution reaction under nearly neutral conditions. In this work, we report the transformation of Ni3S2 nanowire array on nickel foam into the amorphous nickel carbonate nanowire array on nickel foam (NiCO3/NF). The resulting NiCO3/NF shows high electrocatalytic activity towards water oxidation and affords current density of 50 mA∙cm‒2 at overpotential of 395 mV in 1.0 mol∙L‒1 KHCO3. Moreover, this NiCO3/NF is also durable with a long-term electrochemical durability of 60 h. This catalyst electrode achieves a high turnover frequency of 0.21 mol O2∙s‒1 at the overpotential of 500 mV.

Similar content being viewed by others
References
Cook T R, Dogutan D K, Reece S Y, Surendranath Y, Teets T S, Nocera D G. Solar energy supply and storage for the legacy and nonlegacy worlds. Chemical Reviews, 2010, 110(11): 6474–6502
Service R F. Hydrogen cars: Fad or the future? Science, 2009, 324 (5932): 1257–1259
Lin F, Boettcher S W. Adaptive semiconductor/eElectrocatalyst junctions in water-splitting photoanodes. Nature Materials, 2014, 13 (1): 81–86
Walter MG, Warren E L, Mckone J R, Boettcher SW, Mi Q, Santori E S, Lewis N S. Solar water splitting cells. Chemical Reviews, 2010, 110(11): 6446–6473
Zou X, Zhang Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chemical Society Reviews, 2015, 44(15): 5148–5180
Lu X, Gu L, Wang J, Wu J, Liao P, Li G. Bimetal-organic framework derived CoFe2O4/C porous hybrid nanorod arrays as high-performance electrocatalysts for oxygen evolution reaction. Advanced Materials, 2017, 29(3): 1604437
Feng J, Ye S, Xu H, Tong Y, Li G. Design and synthesis of FeOOH/CeO2 heterolayered nanotube electrocatalysts for the oxygen evolution reaction. Advanced Materials, 2016, 28(23): 4698–4703
Feng J, Xu H, Dong Y, Ye S, Tong Y, Li G. FeOOH/Co/FeOOH hybrid nanotube arrays as high-performance electrocatalysts for the oxygen evolution reaction. Angewandte Chemie International Edition, 2016, 55(11): 3694–3698
Hong W, Risch M, Stoerzinger K A, Grimaud A, Suntivich J, Shao-Horn Y. Toward the rational design of non-precious transition metal oxides for oxygen electrocatalysis. Energy & Environmental Science, 2015, 8(5): 1404–1427
Yin Q, Tan J M, Besson C, Geletii Y V, Musaev D G, Kuznetsov A E, Luo Z, Hardcastle K I, Hill C L. A fast soluble carbon-free molecular water oxidation catalyst based on abundant metals. Science, 2010, 328(5976): 342–345
Suntivich J, May K J, Gasteiger H A, Goodenough J B, Shao-Horn Y. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science, 2011, 334(6061): 1383–1385
Han L, Dong S, Wang E. Transition-metal (Co, Ni, and Fe)-based electrocatalysts for the water oxidation reaction. Advanced Materials, 2016, 28(42): 9266–9291
Zhong H, Wang J, Meng F, Zhang X. In situ activating ubiquitous rust towards low-cost, efficient, free-standing, and recoverable oxygen evolution electrodes. Angewandte Chemie, 2016, 128(20): 10091–10095
Zhong H, Li K, Zhang Q, Wang J, Meng F, Wu Z, Yan J, Zhang X. In situ anchoring of Co9S8 nanoparticles on N and S co-doped porous carbon tube as bifunctional oxygen electrocatalysts. NPG Asia Materials, 2016, 8(132): e308
Le Goff A, Artero V, Jousselme B, Tran P D, Guillet N, Métayé R, Fihri A, Palacin S, Fontecave M. From hydrogenases to noble metalfree catalytic nanomaterials for H2 production and uptake. Science, 2009, 326(5958): 1384–1387
Leroy R L. Industrial water electrolysis: Present and future. International Journal of Hydrogen Energy, 1983, 8(83): 401417
Liang H, Meng F, Cabán-Acevedo M, Li L, Forticaux A, Xiu L, Wang Z, Jin S. Hydrothermal continuous flow synthesis and exfoliation of NiCo layered double hydroxide nanosheets for enhanced oxygen evolution catalysis. Nano Letters, 2015, 15(2): 1421–1427
Kanan M W, Nocera D G. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. Science, 2008, 321(5892): 1072–1075
McAlpin J G, Surendranath Y, Dinca M, Stich T A, Stoian S A, Casey W H, Nocera D G, Britt R D. EPR evidence for Co(IV) species produced during water oxidation at neutral pH. Journal of the American Chemical Society, 2010, 132(20): 6882–6883
Esswein A J, Surendranath Y, Reece S Y, Nocera D G. Highly active cobalt phosphate and borate based oxygen evolving catalysts operating in neutrual and natural waters. Energy & Environmental Science, 2011, 4(2): 499–504
Wang W, Liu D, Hao S, Qu F, Ma Y, Du G, Asiri A M, Yao Y, Sun X. High-efficiency and durable water oxidation under mild pH conditions: An iron phosphate-borate nanosheet array as a nonnoble-metal catalyst electrode. Inorganic Chemistry, 2017, 56(6): 3131–3135
Surendranath Y, Kanan M W, Nocera D G. Mechanistic studies of the oxygen evolution reaction by a cobalt-phosphate catalyst at neutral pH. Journal of the American Chemical Society, 2010, 132 (46): 16501–16509
Kanan M W, Yano J, Surendranath Y, Dincă M, Yachandra V K, Nocera D G. Structure and valency of a cobalt-phosphate water oxidation catalyst determined by in situ X-Ray spectroscopy. Journal of the American Chemical Society, 2010, 132(46): 13692–13701
Smith A M, Trotochaud L, Burke M S, Boettcher S W. Contributions to activity enhancement via Fe incorporation in Ni-(oxy)hydroxide/borate catalysts for near-neutral pH oxygen evolution. Chemical Communications (Cambridge), 2015, 51(25): 5261–5263
Dincă M, Surendranath Y, Nocera D G. Nickel-borate oxygenevolving catalyst that functions under benign conditions. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(23): 10337–10341
Bediako D K, Costentin C, Jones E C, Nocera D G, Savéant J M. Proton-electron transport and transfer in electrocatalytic films. Application to a cobalt-based O2-evolution catalyst. Journal of the American Chemical Society, 2013, 135(28): 10492–10502
Bediako D K, Surendranath Y, Nocera D G. Mechanistic studies of the oxygen evolution reaction mediated by a nickel-borate thin film electrocatalyst. Journal of the American Chemical Society, 2013, 135(9): 3662–3674
Yang L, Xie L, Ge R, Kong R, Liu Z, Asiri A M. Core-shell NiFe-LDH@NiFe-Bi nanoarray: In situ electrochemical surface derivation preparation toward efficient water oxidation electrocatalysis in near-neutral media. ACS Applied Materials & Interfaces, 2017, 9 (23): 19502–19506
Kurosu H, Yoshida M, Mastectomy Y, Onishi S, Abe H, Kondoh H. In situ observations of oxygen evolution cocatalysts on photoelec-trodes by X-ray absorption spectroscopy: Comparison between cobalt-phosphate and cobalt-borate. Electrochemistry, 2016, 10(84): 779–783
Joya K S, Takanabe K, de Groot H J M. Surface generation of a cobalt-derived water oxidation electrocatalyst developed in a neutral HCO3-/CO2 system. Advanced Energy Materials, 2014, 4(16): 1400252
Xie F, Wu H, Mou J, Lin D, Xu C, Wu C, Sun X. Ni3N@Ni-Ci nanoarray as a highly active and durable non-noble-metal electrocatalyst for water oxidation at near-neutral pH. Journal of Catalysis, 2017, 356: 165–172
Kanan M W, Nocera D G. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. Science, 2008, 321(5892): 1072–1075
Chen W, Wang H, Li Y, Lee J S, Cui Y. In situ electrochemical oxidation tuning of transition metal disulfides to oxides for enhanced water oxidation. American Chemical Society Central Science, 2015, 1(5): 244–251
Ren Z, Botu V, Wang S, Meng Y, Song W, Guo Y, Ramprasad S, Gao P, Suib S L. Monolithically integrated spinel MxCo3XO4 (M = Co, Ni, Zn) nanoarray catalysts: Scalable synthesis and cation manipulation for tunable low-temperature CH4 and CO oxidation? Angewandte Chemie International Edition, 2014, 53(160): 7223–7227
Kibsgaard J, Chen Z, Reinecke B N, Jaramilo T F. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nature Materials, 2012, 11(11): 963–969
Wang J, Ma M, Qu F, Asiri A M, Sun X. Fe-doped Ni2P nanosheet array for high-efficiency electrochemical water oxidation. Inorganic Chemistry, 2017, 56(3): 1041–1044
He C, Wu X, He Z. Amorphous nickel-based thin film as a janus electrocatalyst for water splitting. Journal of Physical Chemistry C, 2014, 118(9): 4578–4584
Zhu Y, Liu Y, Ren T, Yuan Z. Self-supported cobalt phosphide mesoporous nanorod arrays: A flexible and bifunctional electrode for highly active electrocatalytic water reduction and oxidation. Advanced Functional Materials, 2015, 25(47): 7337–7347
Meng F, Wang Z, Zhong H, Wang J, Yan J, Zhang B. Reactive multifunctional template-induced preparation of Fe-N-doped mesoporous carbon microspheres towards highly efficient electrocatalysts for oxygen reduction. Advanced Materials, 2016, 28(36): 7948–7955
Xie M, Yang L, Ji Y, Wang Z, Ren X, Liu Z, Asiri A M, Xiong X, Sun X. An amorphous Co-carbonate-hydroxide nanowire array for efficient and durable oxygen evolution reaction in carbonate electrolyte. Nanoscale, 2017, 9(43): 16612–16615
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 21575137), the Open Project of State Key Laboratory Cultivation Base for Nonmetal Composites and Functional Materials (No. 16kffk04) and the Key Lab of Process Analysis and Control of Sichuan Universities (No. 2016001). We also appreciate Hui Wang from the Analytical & Testing Center of Sichuan University for her help with SEM characterization.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
11705_2018_1717_MOESM1_ESM.pdf
Nickel-carbonate nanowire array: An efficient and durable electrocatalyst for water oxidation under nearly neutral conditions
Rights and permissions
About this article
Cite this article
Ji, Y., Ma, M., Ji, X. et al. Nickel-carbonate nanowire array: An efficient and durable electrocatalyst for water oxidation under nearly neutral conditions. Front. Chem. Sci. Eng. 12, 467–472 (2018). https://doi.org/10.1007/s11705-018-1717-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11705-018-1717-8