Summary
The metabolic fate of brofaromine (CGP 11 305 A), a new, reversible, selective MAO-A inhibitor, has been assessed in poor (PM) and extensive (EM) metabolizers of debrisoquine.
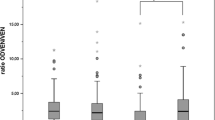
Compared to EM, PM had significantly longer t1/2 (136%) and larger AUC(0−∞) (110%) of the parent compound brofaromine and a lower Cmax (69%) and AUC (0–72 h) (40%) of its O-desmethyl metabolite. The mean metabolite/substrate ratio (based on urine excretion) was about 6-times greater in EM than in PM. Treatment with quinidine converted all EM into phenocopies of PM. All pharmacokinetic parameters of brofaromine and O-desmethyl-brofaromine in EM treated with quinidine were similar to those of untreated PM, including the metabolite/substrate ratio. Quinidine treatment of PM did not alter the pharmacokinetics of brofaromine or of its metabolite, nor the metabolite/substrate ratio.
The results indicate a role for the debrisoquine type of oxidation polymorphism in the O-demethylation and pharmacokinetics of brofaromine.
Similar content being viewed by others
References
Balant PL, Gundert-Remy U, Boobis AR, von Bahr C (1989) Relevance of genetic polymorphism in drug metabolism in the development of new drugs. Eur J Clin Pharmacol 36: 551–554
Bieck P, Antonin KH, Schulz R (1993) Clinical pharmacology of MAO inhibitors. In: Yashura H, Parvez SH, Sandler M, Oguchi K, Nagatsu T (eds) Monoamine oxidase; basic and clinical aspects. VSP Press, Zeist, pp 177–196
Brøsen K, Gram LF, Haghfelt T, Bertilsson L (1987) Extensive metabolizers of debrisoquine become poor metabolizers during quinidine treatment. Pharmacol Toxicol 60: 312–314
Brøsen K, Gram LF (1989) Clinical significance of the sparteine/debrisoquine oxidation polymorphism. Eur J Clin Pharmacol 36: 537–547
Brøsen K (1990) Recent developments in hepatic drug oxidation — implications for clinical pharmacokinetics. Clin Pharmacokinet 18: 220–239
Caporaso NE, Shaw GL (1991) Clinical implications of the competitive inhibition of the debrisoquin-metabolizing isoenzyme by quinidine. Arch Intern Med 151: 1985–1992
Chan K (1988) Comparison of gaschromatographic and high-performance liquid chromatographic assays for the determination of debrisoquine and its 4-hydroxy metabolite in human fluids. J Chromatogr Biomed Appl 425: 311–321
Feifel N, Kucher K, Schmidt E, Antonin KH, Bieck PR (1991) Influence of quinidine on pharmacokinetics and metabolism of the MAO-A inhibitor brofaromine: Role of the debrisoquine-sparteine cytochrome P450 (abstract). Naunyn Schmiedberg's Arch Pharmacol 343 Suppl: R7
Gleiter CH, Aichele G, Nilsson E, Hengen N, Antonin KH, Bieck PR (1985) Discovery of altered pharmacokinetics of CGP 15210 G in poor hydroxylators of debrisoquine during early drug development. Br J Clin Pharmacol 20: 81–84
Guttendorf RJ, Wedlund PJ (1992) Genetic aspects of drug disposition and therapeutics. J Clin Pharmacol 32: 107–117
Idle JR, Oates NS, Sharh RR, Smith RO (1983) Protecting poor metabolisers, a group at high risk of adverse drug reactions. Lancet I: 1388
Mikus G, Ha HR, Vozeh S, Follath F, Eichelbaum M (1986) Pharmacokinetics and metabolism of quinidine in extensive and poor metabolisers of sparteine. Eur J Clin Pharmacol 31: 69–72
Otton SV, Brinn RU, Gram LF (1986) Quinidine 3-hydroxylase activity in human liver microsomes: effect of added debrisoquine, sparteine and desipramine. Acta Pharmac Toxicol 59 [Suppl V]: 113
Schneider W, Keller B, Degen PH (1989) Determination of the new monoamine oxidase inhibitor brofaromine and its major metabolite in biological fluids by gas chromatography with electron-capture detection. J Chromatogr 488: 275–282
Speirs CJ, Murray S, Boobis AR, Seddon CE, Davies DS (1986) Quinidine and the identification of drugs whose elimination is impaired in subjects classified as poor metabolizers of debrisoquine. Br J Clin Pharmacol 22: 739–743
Tyndale RF, Sunahara R, Inaba T, Kalow W, Gonzalez FJ, Niznik HB (1991) Neuronal cytochrome P450IID1 (debrisoquine/sparteine-type): potent inhibition of activity by (-) cocaine and nucleotide sequence identity to human hepatic P450 gene CYP2D6. Mol Pharmacol 40: 63–68
Waldmeier F, Czendlik C, Faigle FW, Moppert J (1988) Abstracts of the XIth European Drug Metabolism Workshop. Konstanz, Germany
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Feifel, N., Kucher, K., Fuchs, L. et al. Role of cytochrome P4502D6 in the metabolism of brofaromine. Eur J Clin Pharmacol 45, 265–269 (1993). https://doi.org/10.1007/BF00315394
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00315394