Abstract
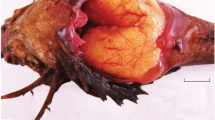
The ovaries of the studied 16 viviparous blenny species of the fish family Clinidae (Teleostei, Perciformes) from Australia and South Africa, are of the cystovarian type and bilobed, ending in a common short oviduct at the gonopore; they are elongated in juvenile fish, becoming bottle-shaped or oval with maturation and onset of embryogenesis, when they comprise 18–20% of the total body weight. The ovarian lumen is covered by the germinal epithelium, resting on a basal membrane. The primordial germ cells are situated along the inner side of each lobe, juxtaposed internally to the lumenal epithelium. Single primary oocytes or nests of such, each in a follicular envelope and covered externally by the lumenal epithelium forming clusters protruding into the lumen, along which the inoculated sperm settle. Two different types of reproductive strategies are demonstrated by the Australian species of Heteroclinus and Cristiceps and the South African species of Clinus and related forms. The Australian species produce high numbers of small (350 ± 50 µm in diameter) eggs that develop synchronously, while the South African species produce much fewer and larger (1.0 ± 0.1 mm) eggs, asynchronous in development. An exception in the latter group is Blennophis striatus, with eggs of ca.1.4 mm that develop synchronously. The Australian species are thus mass releasers that bear and release up to 1000 small (11 ± 1.5 mm in length) embryos/spawn, while the South African Clinus species are discrete releasers that bear eggs of various sizes and small numbers of mature for release embryos that reach up to 22 mm in length, and are released throughout the year. The cytogenesis of the oocytes and surrounding envelopes is discussed.







Similar content being viewed by others
References
Blackburn DG (2005) Evolutionary origins of viviparity in fishes. In: Grier HJ, Uribe MC (eds) Viviparous fishes. New Life Publications, Florida, pp 287–301
Brummett AR, Dumont JN, Larkin JR (1982) The ovary of Fundulus heteroclitus. J Morphol 173:1–16
Devlin RH, Nagahama Y (2002) Sex determination and sex differentiation in fish: an overview of genetic, physiological and environmental influences. Aquaculture 208:191–364
Fishelson L (1978) Oogenesis and spawn formation in the pygmy lionfish Dendrochirus brachypterus. Mar Biol 46:341–348
Fishelson L, Gon O (2008) Comparative oogenesis in cardinal fishes (Apogonidae, Perciformes), with special focus on the adaptive structures of the egg envelopes. Environ Biol Fish 81:397–414
Fishelson L, Gon O, Holdengreber V, Delarea Y (2006) Comparative morphology and cytology of the male sperm-transmission organs in viviparous species of clinid fish (Clinidae, Teleostei). J Morphol 267:1406–1414
Fishelson L, Gon O, Holdengreber V, Delarea Y (2007) Comparative spermatogenesis, spermatocytogenesis, and spermatozeugmata formation in males of viviparous clinid fishes (Teleostei: Clinidae, Blennioidei). Anat Rec 290:311–323
Fraser EA, Renton RM (1941) Observations on the breeding and development of the viviparous fish, Heterandria formosa. Quart J Micr Sci 81:479–520
Gardiner DM (1987) Cyclic changes of the fine structure of the epithelium lining the ovary of the viviparous teleost, Cymatogaster aggregatus (Perciformes: Embiotocidae). J Morphol 156:367–380
Grier H (2000) Ovarian germinal epithelium and folliculogenesis in the common snook, Centropomus undecimalis (Teleostei: Centropomidae). J Morphol 243:265–281
Grier H (2002) The germinal epithelium and its dual role in establishing male reproductive classes and understanding the basis for indeterminate egg production in female fishes. In Croswell RL, editor, Proceedings of the 53rd Annual Gulf and Caribbean Fisheries Institute, Fort Pierce, Florida, pp. 537–552
Grove BD, Wourms JP (1991) The follicular placenta of the viviparous fish, Heterandria formosa. I. Ultrastructure and development of the embryonic absorptive surface. J Morphol 209:265–284
Gunn JS, Thresher RE (1991) Viviparity and reproductive ecology of clinid fishes (Clinidae) from temperate Australian waters. Environ Biol Fish 31:323–344
Lewis ZR, McClellan MC, Postlechwait JH, Cresko WA, Kaplan RH (2008) Female-specific increase in primordial germ cells marks sex differentiation in Three spine Stickleback (Gasterosteus aculeatus). J Morphol 269:909–921
Maack G, Segner H (2003) Morphological development of the gonad in zebrafish. J Fish Biol 64:895–906
Mayer I, Shackley SE, Ryland JS (1988) Aspects of reproductive biology of the bass, Dicentrarchus labrax L. I. A histological and histochemical study of the oocyte development. J Fish Biol 33:609–622
Moser HG (1967) Seasonal histological changes in the gonads of Sebastes paucispinis Ayers, an ovoviviparous teleost (family Scorpaenidae). J Morphol 123:329–354
Moser HG (2007) Reproduction in the viviparous South African clinid fish Fucomimus mus. S Afr J Mar Sci 29:423–436
Parênti LR, Grier HJ (2004) Evolution and phylogeny of gonad morphology in bony fishes. Integr Comp Biol 44:333–348
Patino R, Sullivan CV (2002) Ovarian follicle growth, maturation and ovulation in teleost fishes. Fish Physiol Biochem 26:57–70
Penrith ML (1970) The distribution of fishes of the family Clinidae in South Africa. Ann S Afr Mus 55:1–21
Prochazka K (1994) The reproductive biology of the intertidal klipfish (Perciformes: Clinidae) in South Africa. S Afr J Mar Sci 29:244–253
Prochazka K, Griffiths CL (1992) Observations on the distribution patterns, behavior, diets and reproductive cycles of sand dwelling clinids (Perciformes: Clinidae) from South Africa. Environ Biol Fish 35:371–379
Springer VG (1993) Definition of the suborder Blennioidei and its included families (Pisces: Perciformes). Bull Mar Sci 52:472–495
Ramos MA (2003) Oogenesis in Sparus aurata L. Relat Cient Tec IPIMAR Serie digital 3, 13 pp
Rasmussen TH, Jaspersen A, Korsgaard B (2006) Gonadal morphogenesis and sex differentiation in intraovarian embryos of the viviparous fish Zoarces viviparous (Teleostei, Perciformes, Zoarcidae): A histological and ultrastructural study. J Morphol 267:1032–1047
Tyler CR, Sumpter JP (1996) Oocyte growth and development in teleosts. Rev Fish Biol Fish 6:287–318
Veith WJ (1979) Reproduction in the live-bearing teleost Clinus superciliosus. S Afr J Zool 14:208–211
Veith WJ (1980) Viviparity and embryonic adaptation in the teleost Clinus superciliosus. Can J Zool 58:1–12
Veith WJ, Cornish DA (1986) Ovarian adaptation in the viviparous teleosts Clinus superciliosus and Clinus dorsalis (Perciformes: Clinidae). S Afr J Zool 21:343–347
Wallace RA, Selman K (1981) Cellular and dynamic aspects of oocyte growth in teleosts. Am Zool 21:325–343
Wallace RA, Selman K (1990) Ultrastructural aspects of oogenesis and oocyte growth in fish and amphibians. J Electon Microsc Tech 16:175–201
Wourms JP (1976) Annual fish oogenesis 1. Differentiation of the mature oocyte and formation of the primary envelope. Dev Biol 50:338–354
Wourms JP (1981) Viviparity: The maternal-fetal relationship in fishes. Am Zool 21:472–515
Wourms JP, Collard IP (1992) Evolution of viviparity in vertebrates. Am Zool 32:249–354
Wourms JP, Grove BD, Lombardi J (1988) The maternal-embryonic relationship in viviparous fishes. In: Hoar WS, Randall DJ (eds) Fish physiology, vol. IXB, pp 1–134
Yoshizaki G, Takeuchi Y, Kobayashi T, Ihara S, Takeuchi T (2002) Primordial germ cells: the blueprint for a piscine life. Fish Physiol Biochem 26:3–12
Acknowledgment
The authors are grateful to the following persons and ichthyologic museums of Australia that generously donated the studied fishes: Mark McGrouther, Australian Museum, Sydney; Glenn Moore, Western Australia Museum, Pert; Dianne J. Bray, Museum Victoria. Thanks are due to Yakob Delarea for help with the microsections, Varda Vexler for help with the artwork, and Naomi Paz for editing the draft of this study. The anonymous reviewers contributed important remarks that helped to improve the MS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fishelson, L., Gon, O. Comparison of the ovaries and oogenesis of some Australian and South African viviparid clinid fishes (Clinidae, Blennioidei, Perciformes). Environ Biol Fish 86, 527–540 (2009). https://doi.org/10.1007/s10641-009-9551-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-009-9551-y