Abstract
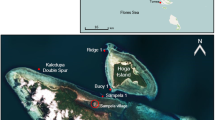
Sponges are an important component of the benthic community, especially on coral reefs, but demographic data such as growth, recruitment or mortality are notably limited. This study examined the growth of the elephant ear sponge Ianthella basta, the largest and in some areas one of the dominating sponge species on Guam and other pacific reefs. We measured growth rates of the natural population on Guam over the course of one year and identified intra-individual growth patterns. Initial sponge sizes ranged from 200 to 35,000 cm2. Specific growth rates ranged from 0.08 to 6.08 with a mean specific growth rate of 1.43 ± 1.29 (SD) year−1. Furthermore, specific growth decreased with sponge size. The age estimate for the largest sponge (1.7 m height × 9.5 m circumference) was ~8 years. Intra-individual growth was mostly apical. This study demonstrated high growth rates, which has notable implications for environmental assessments, management and potential biomedical applications.



Similar content being viewed by others
References
Akaike, H., 1973. Information theory and an extension of the maximum likelihood principle. In Petrov, B. N. & F. Csaki (ed.), Proceedings of the 2nd International Symposium on Information Theory. Akademiai Kiado, Budapest: 267–281.
Ayling, A. L., 1983. Growth and regeneration rates in thinly encrusting Demospongiae from temperate waters. Biological Bulletin, Marine Biological Laboratory, Woods Hole 165: 343–352.
Becerro, M. A., R. W. Thacker, X. Turon, M. J. Uriz & V. J. Paul, 2003. Biogeography of sponge chemical ecology: comparisons of tropical and temperate defenses. Oecologia 135: 91–101.
Bergquist, P. R. & M. Kelly-Borges, 1995. Systematics and biogeography of the genus Ianthella (Demospongiae: Verongida: Ianthellidae) in the South-West Pacific. The Beagle, Records of the Museums and Art Galleries of the Northern Territory 12: 151–176.
Beverton, R. J. H. & S. J. Holt, 1957. On the Dynamics of Exploited Fish Populations. Fisheries Investigations of the Ministry of Agriculture and Fisheries, Food in Great Britain, Series 2, Sea Fish, Vol 19. Facsimile reprint 1993. Chapman & Hall, London.
Brunner, E., H. Ehrlich, P. Schupp, R. Hedrich, S. Hunoldt, M. Kammer, S. Machill, S. Paasch, V. V. Bazhenov, D. V. Kurek, T. Arnold, S. Brockmann, M. Ruhnow & R. Born, 2009. Chitin-based scaffolds are an integral part of the skeleton of the marine demosponge Ianthella basta. Journal of Structural Biology 168: 539–547.
Burnham, K. P. & D. R. Anderson, 2002. Model Selection and Multimodel Inference: A Practical Information-Theoretical Approach. Springer, New York.
Dayton, P. K., G. A. Robilliard, R. T. Paine & L. B. Dayton, 1974. Biological accommodation in the benthic community at McMurdo Sound, Antarctica. Ecological Monographs 44: 105–128.
De Caralt, S., M. J. Uriz & R. H. Wifffels, 2008. Grazing, differential size-class dynamics and survival of the Mediterranean sponge Corticium candelabrum. Marine Ecology Progress Series 360: 97–106.
Diaz, M. C. & K. Rützler, 2001. Sponges: an essential component of Caribbean coral reefs. Bulletin of Marine Science 69: 535–546.
Duckworth, A. R. & C. N. Battershill, 2001. Population dynamics and chemical ecology of New Zealand demospongiae Latrunculia sp nov and Polymastia croceus (Poecilosclerida : Latrunculiidae : Polymastiidae). New Zealand Journal of Marine and Freshwater Research 35: 935–949.
Duffy, J. E., 1992. Host use patterns and demography in a guild of tropical sponge-dwelling shrimps. Marine Ecology Progress Series 90: 127–138.
Ebert, T. A., 1980. Estimating parameters in a flexible growth equation, the Richards function. Canadian Journal of Fisheries and Aquatic Sciences 37: 687–692.
Ehrlich, H., M. Krautter, T. Hanke, P. Simon, C. Knieb, S. Heinemann & H. Worch, 2007a. First evidence of the presence of chitin in skeletons of marine sponges. Part II. Glass sponges (Hexactinellida: Porifera). Journal of Experimental Zoology Part B-Molecular and Developmental Evolution 308B: 473–483.
Ehrlich, H., M. Maldonado, K. D. Spindler, C. Eckert, T. Hanke, R. Born, C. Goebel, P. Simon, S. Heinemann & H. Worch, 2007b. First evidence of chitin as a component of the skeletal fibers of marine sponges Part I. Verongidae (Demospongia: Porifera). Journal of Experimental Zoology Part B: Molecular and Developmental Evolution 308B: 347–356.
Ehrlich, H., M. Ilan, M. Maldonado, G. Muricy, G. Bavestrello, Z. Kljajic, J. L. Carballo, S. Schiaparelli, A. Ereskovsky, P. Schupp, R. Born, H. Worch, V. V. Bazhenov, D. Kurek, V. Varlamov, D. Vyalikh, K. Kummer, V. V. Sivkov, S. L. Molodtsov, H. Meissner, G. Richter, E. Steck, W. Richter, S. Hunoldt, M. Kammer, S. Paasch, V. Krasokhin, G. Patzke & E. Brunner, 2010a. Three-dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part I. Isolation and identification of chitin. International Journal of Biological Macromolecules 47: 132–140.
Ehrlich, H., E. Steck, M. Ilan, M. Maldonado, G. Muricy, G. Bavestrello, Z. Kljajic, J. L. Carballo, S. Schiaparelli, A. Ereskovsky, P. Schupp, R. Born, H. Worch, V. V. Bazhenov, D. Kurek, V. Varlamov, D. Vyalikh, K. Kummer, V. V. Sivkov, S. L. Molodtsov, H. Meissner, G. Richter, S. Hunoldt, M. Kammer, S. Paasch, V. Krasokhin, G. Patzke, E. Brunner & W. Richter, 2010b. Three-dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part II: Biomimetic potential and applications. International Journal of Biological Macromolecules 47: 141–145.
Engel, S. & J. R. Pawlik, 2000. Allelopathic activities of sponge extracts. Marine Ecology-Progress Series 207: 273–281.
Faulkner, D. J., M. K. Harper, M. G. Haygood, C. E. Salomon & E. W. Schmidt, 2000. Symbiotic bacteria in sponges: sources of bioactive substances. In Fusetani, N. (ed.), Drugs from the Sea. Karger, Basel: 107–119.
Garrabou, J. & M. Zabala, 2001. Growth dynamics in four mediterranean demosponges. Estuarine, Coastal and Shelf Science 52: 293–303.
Gompertz, B., 1825. On the nature of the function expressive of human mortality, and on a new mode of determining the value of life contingencies. Philosophical Transactions of the Royal Society of London, Series B 115: 513–585.
Hadas, E., M. Shpigel & M. Ilan, 2009. Particulate organic matter as a food source for a coral reef sponge. Journal of Experimental Biology 212: 3643–3650.
Henkel, T. P. & J. R. Pawlik, 2005. Habitat use by sponge-dwelling brittlestars. Marine Biology 146: 301–313.
Hoppe, W. F., 1988. Growth, regeneration and predation in 3 species of large coral reef sponges. Marine Ecology Progress Series 50: 117–125.
Hultgren, K. M. & J. E. Duffy, 2010. Sponge host characteristics shape the community structure of their shrimp associates. Marine Ecology Progress Series 407: 1–12.
Jayakumar, R., D. Menon, K. Manzoor, S. V. Nair & H. Tamura, 2010. Biomedical applications of chitin and chitosan based nanomaterials—a short review. Carbohydrate Polymers 82: 227–232.
Kelly, M., J. N. A. Hooper, V. Paul, G. Paulay, R. W. M. Van Soest & W. de Weerdt, 2003. Taxonomic inventory of the sponges (Porifera) of the Mariana Islands. Micronesica 35–36: 100–120.
Koopmans, M. & R. H. Wijffels, 2008. Seasonal growth rate of the sponge Haliclona oculata (Demospongiae: Haplosclerida). Marine Biotechnology 10: 502–510.
Maeda, Y., R. Jayakumar, H. Nagahama, T. Furuike & H. Tamura, 2008. Synthesis, characterization and bioactivity studies of novel beta-chitin scaffolds for tissue-engineering applications. International Journal of Biological Macromolecules 42: 463–467.
McMurray, S. E., J. E. Blum & J. R. Pawlik, 2008. Redwood of the reef: growth and age of the giant barrel sponge Xestospongia muta in the Florida Keys. Marine Biology 155: 159–171.
McMurray, S. E., T. P. Henkel & J. R. Pawlik, 2010. Demographics of increasing populations of the giant barrel sponge Xestospongia muta in the Florida Keys. Ecology 91: 560–570.
Munro, M. H. G., J. W. Blunt, R. J. Lake, M. Litaudon, C. N. Battershill & M. J. Page, 1994. From seabed to sickbed: what are the prospects? In Van Soest, R. W. M., T. Van Kempen & J. Braekman (eds), Sponges in Space and Time. AA Balkema, Rotterdam: 395–400.
Navy, U.S.D.o.t., 2010. Guam and CNMI Military Relocation: EIS, Vol. 4: Aircraft Carrier Berthing.
Paulay, G., L. Kirkendale, G. Lambert & C. Meyer, 2002. Anthropogenic biotic interchange in a coral reef ecosystem: a case study from Guam. Pacific Science 56: 403–422.
Pauly, D., 1981. The relationships between gill surface area and growth performance in fish: a generalization of von Bertalanffy’s theory of growth. Meeresforschung 28: 251–282.
Pawlik, J. R., 1998. Coral reef sponges: do predatory fishes affect their distribution? Limnology and Oceanography 43: 1396–1399.
Peters, R. H., 1983. The Ecological Implications of Body Size. Cambridge University Press, Cambridge.
Reiswig, H. M., 1971. In situ pumping activities of tropical Demospongiae. Marine Biology 9: 38–50.
Reiswig, H. M., 1973. Population dynamics of three Jamaican Demospongiae. Bulletin of Marine Science 23: 191–226.
Richards, F. J., 1959. A flexible growth function for empirical use. Journal of Experimental Botany 10: 290–300.
Riisgard, H. U. & P. S. Larsen, 2010. Particle capture mechanisms in suspension-feeding invertebrates. Marine Ecology Progress Series 418: 255–293.
Schmahl, G. P., 1999. Recovery and growth of the giant barrel sponge (Xestospongia muta) following physical injury from a vessel grounding in the Florida Keys. Memoirs of the Queensland Museum 44: 532.
Schupp, P. J., C. Kohlert-Schupp, S. Whitefield, A. Engemann, S. Rohde, T. Hemscheidt, J. M. Pezzuto, T. P. Kondratyuk, E. J. Park, L. Marler, B. Rostama & A. D. Wright, 2009. Cancer chemopreventive and anticancer evaluation of extracts and fractions from marine macro- and micro-organisms collected from twilight zone waters around Guam. Natural Product Communications 4: 1717–1728.
Smith, L. C. & W. H. Hildemann, 1986. Allograft-rejection, autograft fusion and inflammatory responses to injury in Callyspongia diffusa (Porifera, Demospongia). Proceedings of the Royal Society of London, Series B: Biological Sciences 226: 445–464.
Suchanek, T. H., R. C. Carpenter, J. D. Witman & C. D. Harvell, 1985. Sponges as important space competitors in deep Caribbean coral reef communities. In Reaka, M. L. (ed.), The Ecology of Deep and Shallow Coral Reefs. Symposia Series for Undersea Research. NOAA, Rockville: 55–59.
Tanaka, K., 1982. A new growth curve which expresses infinitive increase. Publications of the Amakusa Marine Biology Laboratory Kyushu University 6: 167–177.
Tanaka, K., 2002. Growth dynamics and mortality of the intertidal encrusting sponge Halichondria okadai (Demospongiae, Halichondrida). Marine Biology 140: 383–389.
Targett, N. M. & G. P. Schmahl, 1984. Chemical Ecology and Ddistribution of Sponges in the Salt River Canyon, St. Croix, U.S.V.I. NOAA Technical Memorandum OAR NURP-1.
Turon, X., I. Tarjuelo & M. J. Uriz, 1998. Growth dynamics and mortality of the encrusting sponge Crambe crambe (Poecilosclerida) in contrasting habitats: correlation with population structure and investment in defence. Functional Ecology 12: 631–639.
von Bertalanffy, L., 1938. A quantitative theory of organic growth (inquires on growth laws II). Human Biology 10: 181–213.
Winsor, C., 1932. The Gompertz curve as a new growth curve. Proceedings of the National Academy of Science, USA 18: 1–8.
Wulff, J. L., 1985. Patterns and processes of size change in Caribbean demosponges of branching morphology. In Rutzler, K. (ed.), New Perspectives in Sponge Biology. Smithsonian Institution Press, Washington: 425–435.
Wulff, J. L., 1997. Parrotfish predation on cryptic sponges of Caribbean coral reefs. Marine Biology 129: 41–52.
Acknowledgments
We like to thank Gitta Rohde, Ciemon F. V. Caballes and the UOG Marine Lab Techs for assistance in the field. This research was in part supported by NIH MBRS SCORE grant S06-GM-44796 to PJS. Comments of two anonymous reviewers improved the manuscript. SR was supported by a fellowship within the Postdoc-Program of the German Academic Exchange Service (DAAD).
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: M. Maldonado, X. Turon, M. A. Becerro & M. J. Uriz / Ancient animals, new challenges: developments in sponge research
Rights and permissions
About this article
Cite this article
Rohde, S., Schupp, P.J. Growth and regeneration of the elephant ear sponge Ianthella basta (Porifera). Hydrobiologia 687, 219–226 (2012). https://doi.org/10.1007/s10750-011-0774-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-011-0774-5