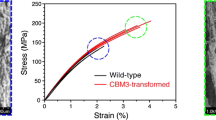
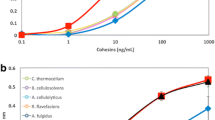
Abstract
Most secondary plant cell walls contain irregular regions known as dislocations or slip planes. Under industrial biorefining conditions dislocations have recently been shown to play a key role during the initial phase of the enzymatic hydrolysis of cellulose in plant cell walls. In this review we chart previous publications that have discussed the structure of dislocations and their susceptibility to hydrolysis. The supramolecular structure of cellulose in dislocations is still unknown. However, it has been shown that cellulose microfibrils continue through dislocations, i.e. dislocations are not regions where free cellulose ends are more abundant than in the bulk cell wall. In more severe cases cracks between fibrils form at dislocations and it is possible that the increased accessibility that these cracks give is the reason why hydrolysis of cellulose starts at these locations. If acid or enzymatic hydrolysis of plant cell walls is carried out simultaneously with the application of shear stress, plant cells such as fibers or tracheids break at their dislocations. At present it is not known whether specific carbohydrate binding modules (CBMs) and/or cellulases preferentially access cellulose at dislocations. From the few studies published so far it seems that no special type of CBM is involved. In one case an endoglucanase was found to preferably bind to dislocations.



Similar content being viewed by others
Abbreviations
- CBM:
-
Carbohydrate binding domain
- GH:
-
Glycoside hydrolase
- MFA:
-
Microfibril angle
References
Akin D, Slomczynski D, Rigsby L, Eriksson K-EL (2002) Retting flax with endopolygalacturonase from Rhizopus oryzae. Textile Res J 72:27–34
Ander P (2002) Dislocations and balloon swelling in spruce kraft pulp fibers—effect of cellulases, xylanase and laccase/HBT. In: Viikari L, Lantto R (eds) Progress in biotechnology 21: biotechnology in the pulp and paper industry: 8th ICBPPI. Elsevier Science BV, Amsterdam, pp 49–59
Ander P, Daniel G, Garcia-Lindgren C, Marklund A (2005) Characterization of industrial and laboratory pulp fibers using HCl, cellulase and fiber master analyses. Nord Pulp Pap Res J 20:115–120
Ander P, Hildén L, Daniel G (2008) Cleavage of softwood kraft pulp fibers by HCl and cellulases. Bioresources 3:477–490
Andersons J, Poriķe E, Spārniņš E (2011) Modeling strength scatter of elementary flax fibers: the effect of mechanical damage and geometrical characteristics. Compos Part A Appl Sci 42:543–549
Aslan M, Chinga-Carrasco G, Sørensen BMB (2011) Strength variability of single flax fibers. J Mater Sci 46:6344–6354
Astley OM, Donald AM (2001) A small-angle X-ray scattering study of the effect of hydration on the microstructure of flax fibers. Biomacromolecules 2:672–680
Atalla R, Brady J, Matthews JF, Ding SY, Himmel M (2008) Structures of plant cell wall celluloses. In: Himmel M (ed) Biomass recalcitrance: deconstructing the plant cell wall for bioenergy. Blackwell Publishing, Oxford, pp 188–212
Baley C (2004) Influence of kink bands on the tensile strength of flax fibers. J Mater Sci 39:331–334
Boraston AB, Kwan E, Chiu P, Warren RAJ, Kilburn DG (2003) Recognition and hydrolysis of noncrystalline cellulose. J Biol Chem 278:6120–6127
Boraston AB, Bolam DN, Gilbert HJ, Davies GJ (2004) Carbohydrate-binding modules: fine-tuning polysaccharide recognition. Biochem J 382:769–781
Bos HL, Donald AM (1999) In situ ESEM study of the deformation of elementary flax fibers. J Mater Sci 34:3029–3034
Bos HL, van den Oever MJA, Peters OCJJ (2002) Tensile and compressive properties of flax fibers for natural fiber reinforced composites. J Mater Sci 34:1683–1692
Buchert J, Tamminen T, Viikari L (1997) Impact of the Donnan effect on the action of xylanases on fiber substrates. J Biotechnol 57:217–222
Buschle-Diller G, Zeronian SH, Pan N, Yoon MY (1994) Enzymatic hydrolysis of cotton, linen, ramie, and viscose rayon fabrics. Textile Res J 64:270–279
Buschle-Diller G, Fanter C, Loth F (1999) Structural changes in hemp fibers as a result of enzymatic hydrolysis with mixed enzyme systems. Textile Res J 69:244–251
Carrard G, Koivula A, Söderlund H, Béquin P (2000) Cellulose-binding domains promote hydrolysis of different sites on crystalline cellulose. Proc Natl Acad Sci 97:10342–10347
Cavaco-Paulo A, Almeida L, Bishop D (1996) Effects of agitation and endoglucanase pretreatment on the hydrolysis of cotton fabrics by a total cellulase. Textile Res J 66:280–294
Chanzy H, Henrissat B (1985) Undirectional degradation of Valonia cellulose microcrystals subjected to cellulase action. FEBS Lett 184:285–288
Cicortas-Gunnarsson L, Montanier C, Tunnicliffe RB, Williamson MP, Gilbert HJ, Nordberg-Karlsson E, Ohlin M (2007) Novel xylan-binding properties of an engineered family 4 carbohydrate-binding module. Biochem J 406:209–214
Clarke K, Li X, Li K (2011) The mechanism of fiber cutting during enzymatic hydrolysis of wood biomass. Biomass Bioenerg 35:3943–3950
Cochaux A, d’Aveni A (1995) Anomalies of structure along cellulosic fibers: characterization and consequences. Revue A T I P49:154–162
Cochaux A, d’Aveni A (1996) Fundamental differences between beating and cellulasic actions of softwood kraft fibers. Revue A T I P 50:49–55
Dai D, Fan M (2011) Investigation of the dislocation of natural fibers by Fourier-transform infrared spectroscopy. Vib Spectrosc 55:300–306
Davies G, Bruce D (1998) Effect of environmental relative humidity and damage on the tensile properties of flax and nettle fibers. Textile Res J 68:623–629
Del Rio LF, Chandra RP, Saddler JN (2012) Fiber size does not appear to influence the ease of enzymatic hydrolysis of organosolv-pretreated softwoods. Bioresour Technol 107:235–242
Ding SY, Xu Q, Ali MK, Baker JO, Bayer EA, Barak Y et al (2006) Versatile derivatives of carbohydrate-binding modules for imaging of complex carbohydrates approaching the molecular level of resolution. Biotechniques 41:435–442
Donaldson LA (1988) Ultrastructure of wood cellulose substrates during enzymatic hydrolysis. Wood Sci Technol 22:33–41
Dunker AK, Fernández A (2007) Engineering productive enzyme confinement. Trends Biotechnol 25:189–190
Eder M, Burgert I (2010) Natural fibers—function in nature. In: Müssiq Jörg (ed) Industrial applications of natural fibers: structure, properties and technical applications, 1st edn. Wiley, New York
Eder M, Terziev N, Daniel G, Burgert I (2008) The effect of (induced) dislocations on the tensile properties of individual Norway spruce fibers. Holzforschung 62:77–81
Fält S, Wågberg L (2003) Influence of electrolytes on the swelling and strength of kraft-liner pulps. Nord Pulp Pap Res J 18(1):69–73
Fernandez EO, Young RA (1996) Properties of cellulose pulps from acidic and basic processes. Cellulose 3:21–44
Filonova L, Gunnarsson LC, Daniel G, Ohlin M (2007a) Synthetic xylan-binding modules for mapping of pulp fibers and wood sections. BMC Plant Biol 7:54
Filonova L, Kallas M, Greffe L, Johansson G, Teeri TT, Daniel G (2007b) Analysis of the surfaces of wood tissues and pulp fibers using carbohydrate-binding modules specific for crystalline cellulose and mannan. Biomacromolecules 8:91–97
Forgacs OL (1961) Structural weakness in softwood pulp tracheids. Tappi J 44:112–119
Frey-Wyssling A (1953) Űber den Feinbau der Stauchlinien in űberbeanspruchtem Holz. Holz als Roh- und Werkstoff 11(7):283–287
Frölander U, Hartler N, Nyren J (1969) Misaligned zones in cellulosic fibers. Part 4. Their influence on the rate of acid hydrolysis. Cell Chem Tech 3:499–506
Gama FM, Teixeira JA, Mota M (1994) Cellulose morphology and enzymatic reactivity: a modified solute exclusion technique. Biotech Bioeng 43:381–387
Grethlein HE (1985) The effect of pore size distribution on the rate of enzymatic hydrolysis of cellulosic substrates. Biotechnology 3:155–160
Grignon J, Scallan AM (1980) Effect of pH and neutral salts upon the swelling of cellulose gels. J Appl Polym Sci 25:2829–2843
Gurnagul N, Page DH, Paice MG (1992) The effect of cellulose degradation on the strength of wood pulp fibers. Nord Pulp Pap Res J 3:152–154
Hartler N (1995) Aspects on curled and microcompressed fibers. Nord Pulp Pap Res J 10:4–7
Henshaw JL, Bolam DN, Pires VM, Czjzek M, Henrissat B, Ferreira LM, Fontes CM, Gilbert HJ (2004) The family 6 carbohydrate binding module CmCBM6–2 contains two ligand-binding sites with distinct specificities. J Biol Chem 279:21552–21559
Hermans PH, Weidinger A (1949) X-ray studies on the crystallinity of cellulose. J Polym Sci 4:135–144
Hildén L, Daniel G, Johansson G (2003) Use of a fluorescence labelled, carbohydrate-binding module from Phanerochaete chrysosporium Cel7D for studying wood cell wall ultrastructure. Biotechnol Lett 25:553–558
Hildén L, Väljamäe P, Johansson G (2005) Surface character of pulp fibers studied using endoglucanases. J Biotechnol 118:386–397
Hoffmeyer P (1990) Failure of wood as influenced by moisture and duration of load. PhD thesis, The State University of New York, College of Environmental Science and Forestry
Iribarne J, Schroeder LR (1999) The use of fiber cut probabilities to study fiber weak points. TAPPI international paper physics conference, San Diego, USA, pp 259–288
Kawakubo T, Karita S, Araki Y, Watanabe S, Oyadomari M, Takada R et al (2010) Analysis of exposed cellulose surfaces in pretreated wood biomass using carbohydrate-binding module (CBM)-cyan fluorescent protein (CFP). Biotechnol Bioeng 105:499–508
Keckes J, Burgert I, Frűhmann K, Műller M, Kölln K, Hamilton M et al (2003) Cell-wall recovery after irreversible deformation of wood. Nat Maters 2:810–813
Khalili S, Akin D, Pettersson B, Henriksson G (2002) Fibernodes in flax and other bast fibers. J Appl Bot 76:133–138
Kibblewhite RP (1976) Fractures and dislocations in the walls of kraft and bisulphite pulp fibers. Cellul Chem Technol 10:497–503
Klyosov AA (1990) Trends in biochemistry and enzymology of cellulose degradation. Biochemistry 29:10577–10585
Koch G, Bauch J, Dűnisch O, Seehann G, Schmitt U (1996) SekundreVer nderungen im Holz akut belasteter Fichten (Picea abies [L.] Karst.) in Hochlagen des Osterzgebirges. Hotz als Roh- und Werkstoff 54:243–249
Lai-Kee-Him J, Chanzy H, Műller M, Putaux JL, Imai T, Bulone V (2002) In vitro versus in vivo cellulose microfibrils from plant primary wall synthases: structural differences. J Biol Chem 277:36931–36939
Laine C, Wang X, Tenkanen M, Varhimo A (2004) Changes in the fiber wall during refining of bleached pine kraft pulp. Holzforschung 58:233–240
Lee SB, Kim IH, Ryu DD, Taguchi H (1983) Structural properties of cellulose and cellulase reaction mechanism. Biotechnol Bioeng 25:33–51
Lehtiö J, Sugiyama J, Gustavsson M, Fransson L, Linder M, Teeri TT (2003) The binding specificity and affinity determinants of family 1 and family 3 cellulose binding modules. Proc Natl Acad Sci 100(2):484–489
LeMoigne N, Spinua M, Heinzeb T, Navarda P (2010) Restricted dissolution and derivatization capacities of cellulose fibers under uniaxial elongational stress. Polymer 51:447–453
Lenting HBM, Warmoeskerken M (2001) Mechanism of interaction between cellulase action and applied shear force, an hypothesis. J Biotechnol 89:217–226
Mansfield SD, Mooney C, Saddler JN (1999) Substrate and enzyme characteristics that limit cellulose hydrolysis. Biotechnol Prog 15:804–816
Nishiyama Y, Kim U-J, Kim D-Y, Katsumata KS, May RP, Langan P (2003) Periodic disorder along ramie cellulose microfibrils. Biomacromolecules 4:1013–1017
Nyholm K, Ander P, Bardage S, Daniel G (2001) Dislocations in pulp fibers-their origin, characteristics and importance-a review. Nord Pulp Pap Res J 16:376–384
O’Sullivan AC (1997) Cellulose: the structure slowly unravels. Cellulose 4:173–207
Park S, Venditti RA, Abrecht DG, Jameel H, Pawlak JJ, Lee JM (2007) Surface and pore structure modification of cellulose fibers through cellulase treatment. J Appl Polym Sci 103:3833–3839
Park S, Baker J, Himmel M, Parilla P, Johnson D (2010) Cellulose crystallinity index: measurement techniques and their impact on interpreting cellulase performance. Biotechnol Biofuels 3:10
Peura M, Saren MP, Laukkanen J, Nygard K, Andersson S, Saranpää P et al (2008) The elemental composition, the microfibril angle distribution and the shape of the cell cross-section in Norway spruce xylem. Trees 22:499–510
Quirk A, Lipkowski J, Vandenende C, Cockburn D, Clarke AJ, Dutcher JR, Roscoe SG (2010) Direct visualization of the enzymatic digestion of a single fiber of native cellulose in an aqueous environment by atomic force microscopy. Langmuir 26:5007–5013
Rao S (2009) Enzymatic hydrolysis of cellulosic fiber. Master thesis, Georgia Institute of Technology, USA
Reese E, Segal L, Tripp V (1957) The effect of cellulase on the degree of polymerization of cellulose and hydrocellulose. Textile Res J 27:626–632
Roberts E, Bose JL, Rowland S (1972) Evidence of two types of accessible surfaces in fibrous cotton. Textile Res J 42:217–221
Robinson W (1920) The microscopical features of mechanical strains in timber and the bearing on these on the structure of the cell-walls in plants. Phil Trans R Soc B210:49–82
Rouvinen J, Bergfors T, Teeri T, Knowles J, Jones A (1990) Three-dimensional structure of cellobiohydrolase II from Trichoderma reesei. Science 249:380–386
Samaniuk JR, Scott CT, Root TW, Klingenberg DJ (2011) The effect of high intensity mixing on the enzymatic hydrolysis of concentrated cellulose fiber suspensions. Bioresour Technol 102:4489–4494
Selby K (1961) The degradation of cotton cellulose by the extracellular cellulase of Myrothecium verrucaria. Biochem J 79:562–566
Selby K, Maitland CC (1967) The cellulase of Trichoderma viride: separation of the components involved in the solubilization of cotton. Biochem J 104:716–724
Suchy M, Hakala T, Kangas H, Kontturi E, Tammelin T, Pursula T, Vuorinen T (2009) Effects of commercial cellobiohydrolase treatment on fiber strength and morphology of bleached hardwood pulp (10th EWLP, Stockholm, Sweden, August 25–28, 2008). Holzforschung 63:731–736
Suurnäkki A (1996) Hemicelulases in the bleaching and characterisation of kraft pulps. Ph D thesis, Technical Research Centre of Finland, Espoo, Finland
Terziev N, Daniel G, Marklund A (2005) Dislocations in Norway spruce fibers and their effect on properties of pulp and paper. Holzforschung 59:163–169
Thygesen LG (2008) Quantification of dislocations in hemp fibers using acid hydrolysis and fiber segment length distributions. J Mater Sci 43:1311–1317
Thygesen LG, Asgharipour MR (2008) The effects of growth and storage conditions on dislocations in hemp fibers. J Mater Sci 43:3670–3673
Thygesen LG, Bilde-Sørensen JB, Hoffmeyer P (2006) Visualisation of dislocations in hemp fibers: a comparison between scanning electron microscopy (SEM) and polarized light microscopy (PLM). Ind Crop Prod 24:181–185
Thygesen LG, Eder M, Burgert I (2007) Dislocations in single hemp fibers: investigations into the relationship of structural distortions and tensile properties at the cell wall level. J Mater Sci 42:558–564
Thygesen LG, Hidayat BJ, Johansen KS, Felby C (2011) Role of supramolecular cellulose structures in enzymatic hydrolysis of plant cell walls. J Ind Microbiol Biotechnol 38:975–983
Vainio U, Andersson S, Serimaa R, Paakkari T, Saranpää P, Treacy M, Evertsen J (2002) Variation of microfibril angle between four provenances of Sitka spruce (Picea sitchensis [Bong.] Carr.). Plant Biol 4:27–33
Wallace JT (2006) An enzymatic fiber modification method for enhancing tissue properties. Master thesis, North Carolina State University, USA
Wardrop AB, Jutte SM (1968) The enzymatic degradation of cellulose from Valonia ventricosa. Wood Sci Technol 2:105–114
Weimar PJ, Weston WM (1985) Relationship between the fine structure of native cellulose and cellulose degradability by the cellulase complexes of Trichoderma reesei and Clostridium thermocellum. Biotechnol Bioeng 27:1540–1547
White AR, Brown RM Jr (1981) Enzymatic hydrolysis of cellulose: visual characterization of the process. Proc Natl Acad Sci 78:1047–1051
Acknowledgments
The authors gratefully acknowledge the financial support from Novozymes A/S, Denmark.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hidayat, B.J., Felby, C., Johansen, K.S. et al. Cellulose is not just cellulose: a review of dislocations as reactive sites in the enzymatic hydrolysis of cellulose microfibrils. Cellulose 19, 1481–1493 (2012). https://doi.org/10.1007/s10570-012-9740-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-012-9740-2