Summary
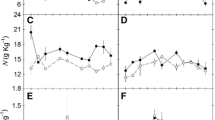
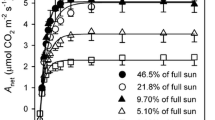
The characteristics of the photosynthetic apparatus of 11 Hawaiian Euphorbia species, all of which possess C4 photosynthesis but range from arid habitat, drought-deciduous shrubs to mesic or wet forest evergreen trees and shrubs, were investigated under uniform greenhouse conditions. Nine species exhibited CO2 response curves typical of C4 plants, but differed markedly in photosynthetic capacity. Light-saturated CO2 uptake rates ranged from 48 to 52 μmol m-2 s-1 in arid habitat species to 18 to 20 μmol m-2 s-1 in mesic and wet forest species. Two possessed unusual CO2 response curves in which photosynthesis was not saturated above intercellular CO2 pressures [p(CO2)] of 10 to 15 Pa, as typically occurs in C4 plants.
Both leaf (g′1) and mesophyll (g′m) conductances to CO2 varied widely between species. At an atmospheric p(CO2) of 32 Pa, g′1 regulated intercellular p(CO2) at 12–15 Pa in most species, which supported nearly maximum CO2 uptake rates, but did not result in excessive transpiration. Intercellular p(CO2) was higher in the two species with unusual CO2 response curves. This was especially apparent in E. remyi, which is native to a bog habitat. The regulation of g′1 and intercellular p(CO2) yielded high photosynthetic water use efficiencies (P/E) in the species with typical CO2 response curves, whereas P/E was much lower in E. remyi.
Photosynthetic capacity was closely related to leaf nitrogen content, whereas correlations with leaf morphological characteristics and leaf cell surface area were not significant. Thus, differences in photosynthetic capacity may be determined primarily by investment in the biochemical components of the photosynthetic apparatus rather than by differences in diffusion limitations. The lower photosynthetic capacities in the wet habitat species may reflect the lower light availability. However, other factors, such as reduced nutrient availability, may also be important.
Similar content being viewed by others
References
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Björkman O (1973) Comparative studies on photosynthesis in higher plants. In: A Geise (ed) Photophysiology, Vol 8. Academic Press, New York, p 1–63
Björkman O (1976) Adaptive and genetic aspects of C4 photosynthesis. In: RH Burris, CC Black (eds) CO2 Metabolism and Plant Productivity. Univ Park Press, Baltimore, p 287–309
Björkman O, Boynton J, Berry J (1976) Comparison of the heat stability of photosynthesis, chloroplast membrane reactions, photosynthetic enzymes and soluble protein in leaves of heatadapted and cold-adapted C4 species. Carnegie Inst Washington Yearb 75:400–407
Bloom AJ, Mooney HA, Björkman O, Berry J (1980) Materials and methods for carbon dioxide and water vapor exchange analysis. Plant Cell Env 3:371–376
Blumenstock DI, Price S (1967) Climates of the states, Hawaii. US Dept of Commerce, 27 p
Chabot BF, Chabot JF (1977) Effects of light and temperature on leaf anatomy and photosynthesis in Fragaria vesica. Oecologia (Berl) 26:363–377
Cowan IR (1977) Stomatal behaviour and environment. Adv Bot Res 4:117–228
Croizat L, Degener O (1936) Chamaesyce. In: O Degener (ed) Flora Hawaiiensis. Privately published
Doliner LH, Jolliffe PA (1979) Ecological evidence concerning the adaptive significance of the C4 dicarboxylic acid pathway of photosynthesis. Oecologia (Berl) 38:23–34
Ehleringer JR, Björkman O (1977) Quantum yields for CO2 uptake in C3 and C4 plants: dependence on temperature, CO2, and O2 concentration. Plant Physiol 59:86–90
Ehleringer JR, Björkman O (1978) A comparison of photosynthetic characteristics of Encelia species possessing glabrous and pubescent leaves. Plant Physiol 62:185–190
Ehleringer JR, Pearcy RW (1982) Variations in quantum yields among C4 plants (In preparation)
Fosberg FR (1948) Derivation of the flora of the Hawaiian Islands. In: EC Zimmerman (ed) Insects of Hawaii, Volume 1. Univ Hawaii Press, Honolulu, p 107–119
Gifford RM (1974) A comparison of potential photosynthesis, productivity and yield of plant species with differeing photosynthetic metabolism. Aust J Plant Physiol 1:107–117
Hawaii Water Authority (1959) Water Resources in Hawaii, 148 p
Hozumi K, Yoda K, Kira T (1969) Production ecology of tropical rainforests in southwestern Cambodia, II. Photosynthetic production in an evergreen seasonal forest. Nature Life Southeast Asia 6:57–81
Jarvis P (1971) The estimation of resistances to carbon dioxide transfer. In: Z. Sestak, J Catsky, PG Jarvis (eds) Plant photosynthetic production: Manual of methods. Junk, The Hague, p 566–631
Kennedy RA, Laetsch RM (1974) Plant species intermediate for C3, C4 photosynthesis. Science 184:1087–1089
Laetsch WM (1974) The C4 syndrome: a structural analysis. Ann Rev Plant Physiol 25:27–52
Longstreth DJ, Hartsock TL, Nobel PS (1980) Mesophyll cell properties for some C3 and C4 species with high photosynthetic rates. Physiol Plant 48:494–498
Mooney HA, Ferrar PJ, Slatyer RO (1978) Photosynthetic capacity and carbon allocation patterns in diverse growth forms of Eucalyptus. Oecologia (Berl) 36:103–111
Morgan JA, Brown RH (1979) Photosynthesis in grass species differeing in carbon dioxide fixation pathways. II. A search for species with intermediate gas exchange and anatomical characteristics. Plant Physiol 64:257–262
Nobels PS (1977) Internal leaf area and cellular CO2 resistance: pohotosynthetic implications of variations with growth conditions and plant species. Physiol Plant 40:137–144
Pearcy RW, Troughton J (1975) C4 photosynthesis in tree form Euphorbia species from Hawaiian rainforest sites. Plant Physiol 55:1054–1056
Pearcy RW, Tumosa N, Williams K (1981) Relationships between growth, photosynthesis and competitive interactions for a C3 and a C4 plant. Oecologia (Berl) 48:371–376
Robichaux RH, Pearcy RW (1980) Environmental characteristics, field water relations, and photosynthetic responses of C4 Hawaiian Euphorbia species from contrasting habitats. Oecologia (Berl) 47:99–105
Teeri JA (1979) The climatology of the C4 photosynthetic pathway. In: OT Solbrig, S Jain, GB Johnson, RH Raven (eds) Topics in plant population biology. Univ Press, Columbia, p 356–374
Tieszen LL, Senyimba MM, Imbamba SK, Troughton JH (1979) The distribution of C3 and C4 grasses and carbon isotope discrimination along an altitudinal and moisture gradient in Kenya. Oecologia (Berl) 37:337–350
Weibel ER (1969) Stereological prinicples for morphometry in electron microscopic cytology. Int Rev Cytol 26:235–302
Wilson JR (1975) Comparative response to nitrogen deficiency of a tropical and temperate grass in the interrelation between photosynthesis, growth, and the accumulation of non-structural carbohydrate. Neth J Agric Sci 23:104–112
Wong SC, Cowan IR, Farquhar GD (1978) Leaf conductance in relation to assimilation in Eucalyptus pauciflora sieb. ex spreng. Plant Physiol 62:670–674
Wong SC, Cowan IR, Farquhar GD (1979) Stomatal conductance correlates with photosynthetic capacity. Nature 282:424–426
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pearcy, R.W., Osteryoung, K. & Randall, D. Carbon dioxide exchange characteristics of C4 Hawaiian Euphorbia species native to diverse habitats. Oecologia 55, 333–341 (1982). https://doi.org/10.1007/BF00376921
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00376921