Abstract
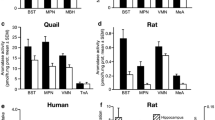
The transformation of testosterone into estradiol in the brain plays a key role in several behavioral and physiological processes, but it has been so far impossible to localize precisely the cells of the mammalian brain containing the relevant enzyme, viz., aromatase. We have recently established an immunohistochemical technique that allows the visualization of aromatase-immunoreactive cells in the quail brain. In this species, a marked increase in the optical density of aromatase-immunoreactive cells is observed in subjects that have been treated with the aromatase inhibitor, R76713 or racemic Vorozole. This increased immunoreactivity, associated with a total blockade of aromatase activity, has been used as a tool in the present study in which the distribution of aromatase-immunoreactive material has been reassessed in the brain of mice pretreated with R76713. As expected, the aromatase inhibitor increases the density of the immunoreactive signal in mice. Strongly immunoreactive cells are found in the lateral septal region, the bed nucleus of the stria terminalis, the central amygdala, and the dorso-lateral hypothalamus. A less dense signal is also present in the medial preoptic area, the nucleus accumbens, several hypothalamic nuclei (e.g., paraventricular and ventromedial nuclei), all divisions of the amygdala, and several regions of the cortex, especially the cortex piriformis. These data demonstrate that, contrary to previous claims, aromatase-immunoreactive cells are present in all brain regions that have been shown previously to contain high aromatase activity.
Similar content being viewed by others
References
Balthazart J (1989) Steroid metabolism and the activation of social behavior. In: Balthazart J (ed) Advances in comparative and environmental physiology, vol 3. Springer, Berlin Heidelberg New York, pp 105–159
Balthazart J, Evrard L, Surlemont C (1990a) Effects of the nonsteroidal aromatase inhibitor, R76713 on testosterone-induced sexual behavior in the Japanese quail (Coturnix coturnix japonica). Horm Behav 24:510–531
Balthazart J, Foidart A, Harada N (1990b) Immunocytochemical localization of aromatase in the brain. Brain Res 514:327–333
Balthazart J, Foidart A, Surlemont C, Vockel A, Harada N (1990c) Distribution of aromatase in the brain of the Japanese quail, ring dove, and zebra finch: an immunocytochemical study. J Comp Neurol 301:276–288
Balthazart J, Foidart A, Surlemont C, Harada N (1991) Distribution of aromatase-immunoreactive cells in the mouse forebrain. Cell Tissue Res 263:71–79
Balthazart J, Foidart A, Surlemont C, Harada N, Naftolin F (1992) Neuroanatomical specificity in the autoregulation of aromatase-immunoreactive neurons by androgens and estrogens: an immunocytochemical study. Brain Res 574:280–290
Bellino FL, Tseng L, Lobo JO (1987) Antisera against estrogen synthetase from human placental microsomes. Antibody characterization and cross-reactivity studies in other organs. Mol Cell Endocrinol 52:143–150
Callard GV (1984) Aromatization in brain and pituitary: an evolutionary perspective. In: Celotti F, Naftolin F, Martini L (eds) Metabolism of hormonal steroids in the neuroendocrine structures. Raven Press, New York, pp 79–102
Callard GV, Petro Z, Ryan KJ (1978a) Conversion of androgen to estrogen and other steroids in the vertebrate brain. Am Zool 18:511–523
Callard GV, Petro Z, Ryan KJ (1978b) Phylogenetic distribution of aromatase and other androgen-converting enzymes in the central nervous system. Endocrinology 103:2283–2290
Celotti F, Massa R, Martini L (1979) Metabolism of sex steroids in the central nervous system. In: DeGroot LJ (ed) Endocrinology. Grune & Stratton, New York, pp 41–53
De Coster R, Wouters W, Bowden CR, Vanden Bossche H, Bruynseels J, Tuman RW, Van Ginckel R, Snoeck E, Van Peer A, Janssen PAJ (1990) New non-steroidal aromatase inhibitors: focus on R76713. J Steroid Biochem 37:335–341
Foidart A, Harada N, Balthazart J (1994a) Effects of steroidal and non steroidal aromatase inhibitors on sexual behavior and aromatase-immunoreactive cells and fibers in the quail brain. Brain Res 657:105–123
Foidart A, Reid J, Yoshimura N, Harada N, Balthazart J (1994b) Identification of aromatase immunoreactive cells in the quail brain by antibodies raised against recombinant quail, mouse and human aromatases. Soc Neurosci Abstr 20:95
Harada N (1988) Novel properties of human placental aromatase as cytochrome P-450: purification and characterization of a unique form of aromatase. J Biochem 103:106–113
Jakab RL, Harada N, Naftolin F (1993a) Projective septal neurons are immunoreactive for the estrogen-synthesizing enzyme aromatase. Are there “estrogenergic” neural pathways in the brain. Soc Neurosci Abstr 19:1442
Jakab RL, Horvath TL, Leranth C, Harada N, Naftolin F (1993b) Aromatase immunoreactivity in the rat brain: gonadectomy-sensitive hypothalamic neurons and an unresponsive “limbic ring” of the lateral septum-bed nucleus-amygdala complex. J Steroid Biochem Mol Biol 44:481–498
Kobayashi RM, Reed KC (1977) Conversion of androgens to estrogens (aromatization) in discrete regions of the rat brain: sexual differences and effects of castration. Soc Neurosci Abstr 3:348
Lauber ME, Lichtensteiger W (1994) Pre- and postnatal ontogeny of aromatase cytochrome P450 messenger ribonucleic acid expression in the male rat brain studied by in situ hybridization. Endocrinology 135:1661–1668
Luttge WG (1979) Endocrine control of mammalian male sexual behavior: an analysis of the potential role of testosterone metabolites. In: Beyer C (ed) Endocrine control of sexual behavior. Raven Press, New York, pp 341–363
MacLusky NJ, Naftolin F (1981) Sexual differentiation of the central nervous system. Science 211:1294–1303
MacLusky NJ, Philip A, Hurlburt C, Naftolin F (1984) Estrogen metabolism in neuroendocrine structures. In: Celotti F, Naftolin F, Martini L (eds) Metabolism of hormonal steroids in the neuroendocrine structures. Raven Press, New York, pp 103–116
MacLusky NJ, Naftolin F, Goldman-Rakic PS (1986) Estrogen formation and binding in the cerebral cortex of the developing rhesus monkey. Proc Natl Acad Sci USA 83:513–516
MacLusky NJ, Clark AS, Naftolin F, Goldman-Rakic PS (1987) Estrogen formation in the mammalian brain: possible role of aromatase in sexual differentiation of the hippocampus and neocortex. Steroids 50:459–474
McEwen BS (1981) Neural gonadal steroid actions. Science 211:1303–1311
McEwen BS, Krey LC (1984) Properties of estrogen-sensitive neurons: aromatization, progestin receptor induction and neuroendocrine effects. In: Celotti F, Naftolin F, Martini L (eds) Metabolism of hormonal steroids in the neuroendocrine structures. Raven Press, New York, pp 117–128
Mendelson CR, Wright EE, Evans CT, Porter JC, Simpson ER (1985) Preparation and characterization of polyclonal and monoclonal antibodies against human aromatase cytochrome P-450 (P-450-Arom), and their use in its purification. Arch Biochem Biophys 243:480–491
Naftolin F, Ryan KJ, Petro Z (1972) Aromatization of androstenedione by the anterior hypothalamus of adult male and female rats. Endocrinology 90:295–298
Naftolin F, Ryan KJ, Davies IJ, Reddy VV, Flores F, Petro Z, Kuhn M, White RJ, Takaoka Y, Wolin L (1975) The formation of estrogens by central neuroendocrine tissues. Recent Prog Horm Res 31:295–319
Naftolin F, Leranth C, Balthazart J (1990) Ultrastructural localization of aromatase immunoreactivity in hypothalamic neurons (abstract). The Endocrine Society Bethesda, Maryland, p 192
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates. Academic Press, New York
Pfaff D, Keiner M (1973) Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J Comp Neurol 151:121–158
Roselli CE (1991) Sex differences in androgen receptors and aromatase activity in microdissected regions of the rat brain. Endocrinology 128:1310–1316
Roselli CE, Resko JA (1989) Testosterone regulates aromatase activity in discrete brain areas of male rhesus macaques. Biol Reprod 40:929–934
Roselli CE, Ellinwood WE, Resko JA (1984) Regulation of brain aromatase activity in rats. Endocrinology 114:192–200
Roselli CE, Horton LE, Resko JA (1985) Distribution and regulation of aromatase activity in the rat hypothalamus and limbic system. Endocrinology 117:2471–2477
Roselli CE, Abdelgadir SE, Lephart ED, McPhaul MJ, Resko JA (1993) Androgens regulate aromatase cytochrome P450 mRNA in rat brain. Soc Neurosci Abstr 19:171
Sanghera MK, Simpson ER, McPhaul MJ, Kozlowski G, Conley AJ, Lephart ED (1991) Immunocytochemical distribution of aromatase cytochrome P450 in the rat brain using peptide-generated polyclonal antibodies. Endocrinology 129:2834–2844
Sar M, Stumpf WE (1973) Autoradiographic localization of radioactivity in the rat brain after the injection of 1,2-3H-testosterone. Endocrinology 92:251–256
Schleicher G, Stumpf WE, Morin JK, Drews U (1986) Sites of aromatization of [3H]testosterone in the forebrain of male, female and androgen receptor-deficient Tfm mice: an autoradiographic study. Brain Res 397:290–296
Schlinger BA, Callard GV (1989) Localization of aromatase in synaptosomal and microsomal subfractions of quail (Coturnix coturnix japonica) brain. Neuroendocrinology 49:434–441
Schumacher M, Balthazart J (1987) Neuroanatomical distribution of testosterone metabolizing enzymes in the Japanese quail. Brain Res 422:137–148
Selmanoff MK, Brodkin LD, Weiner RI, Siiteri PK (1977) Aromatization and 5α-reduction of androgens in discrete hypothalamic and limbic regions of the male and female rat. Endocrinology 101:841–848
Sheridan PJ (1978) Localization of androgen- and estrogen-concentrating neurons in the diencephalon and telencephalon of the mouse. Endocrinology 103:1328–1334
Sheridan PJ, Melgosa RT (1983) Aromatization of testosterone to estrogen varies between strains of mice. Brain Res 273:285–289
Shinoda K, Sakamoto N, Osawa Y, Pearson J (1989a) Aromatase neurons in the monkey forebrain demonstrated by antibody against human placental antigen X-P2. Soc Neurosci Abstr 15:232
Shinoda K, Shiotani Y, Osawa Y (1989b) “Necklace olfactory glomeruli” form unique components of the rat primary olfactory system. J Comp Neurol 284:362–373
Shinoda K, Yagi H, Fujita H, Osawa Y, Shiotani Y (1989c) Screening of aromatase-containing neurons in rat forebrain: an immunohistochemical study with antibody against human placental antigen X-P2 (hPAX-P2). J Comp Neurol 290:502–515
Shinoda K, Mori S, Ohtsuki T, Osawa Y (1992) An aromatase-associated cytoplasmic inclusion, the “stigmoid body,” in the rat brain. I. Distribution in the forebrain. J Comp Neurol 322:360–376
Shinoda K, Nagano M, Osawa Y (1993) An aromatase-associated cytoplasmic inclusion, the “stigmoid body,” in the rat brain. II. Ultrastructure (with a review of its history and nomenclature). J Comp Neurol 329:1–19
Sidman RL, Angevine JB, Taber Pierce E (1971) Atlas of the mouse brain and spinal cord. Harvard University Press, Cambridge, Mass
Simerly RB, Chang C, Muramatsu M, Swanson LW (1990) Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol 294:76–95
Somogyi P, Takagi H (1982) A note on the use of picric acid-paraformaldehyde-glutaraldehyde fixative for correlated light and electron microscopic immunocytochemistry. Neuroscience 7:1779–1784
Steimer T (1988) Aromatase activity in rat brain synaptosomes. Is an enzyme associated with the neuronal cell membrane involved in mediating non-genomic effects of androgens? Eur J Neurosci [Suppl]9
Steimer T, Hutchison JB (1981) Androgen increases formation of behaviourally effective oestrogen in dove brain. Nature 292:345–347
Sternberger LA (1979) Immunocytochemistry. Wiley, New York
Stumpf WE (1970) Estrogen-neurons and estrogen-neuron systems in the periventricular brain. Am J Anat 129:207
Stumpf WE, Sar M (1976) Steroid hormone target sites in the brain: the differential distribution of estrogen, progestin, androgen and glucocorticosteroid. J Steroid Biochem 7:1163–1170
Tsuruo Y, Ishimura K, Fujita H, Osawa Y (1994) Immunocytochemical localization of aromatase-containing neurons in the rat brain during pre- and postnatal development. Cell Tissue Res 278:29–39
Vockel A, Pröve E, Balthazart J (1990a) Effects of castration and testosterone treatment on the activity of testosterone-metabolizing enzymes in the brain of male and female zebra finches. J Neurobiol 21:808–825
Vockel A, Pröve E, Balthazart J (1990b) Sex- and age-related differences in the activity of testosterone-metabolizing enzymes in microdissected nuclei of the zebra finch brain. Brain Res 511:291–302
Wagner CK, Morrell JI (1994) The distribution of aromatase mRNA in the brain of adult male and female rats using in situ hybridization. Soc Neurosci Abstr 20:1740
Wouters W, De Coster R, Krekels M, Van Dun J, Beerens D, Haelterman C, Raeymaekers A, Freyne E, Van Gelder J, Venet M, Janssen PAJ (1989) R76713, a new specific non-steroidal aromatase inhibitor. J Steroid Biochem 32:781–788
Wozniak A, Hutchison RE, Hutchison JB (1992) Localisation of aromatase activity in androgen target areas of the mouse brain. Neurosci Lett 146:191–194
Zeier H, Karten HJ (1971) The archistriatum of the pigeon: organization of afferent and efferent connections. Brain Res 31:313–326
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Foidart, A., Harada, N. & Balthazart, J. Aromatase-immunoreactive cells are present in mouse brain areas that are known to express high levels of aromatase activity. Cell Tissue Res 280, 561–574 (1995). https://doi.org/10.1007/BF00318360
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00318360