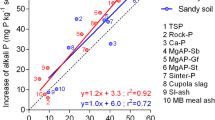
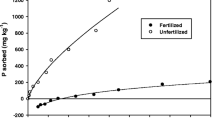
Abstract
Six phosphate (P) compounds, including soil minerals and fertilizer residues, were extracted with eight standard soil P-test reagents. In the absence of soil, the solubility of P differed greatly depending upon P compound type and extractant. For example an aluminium phosphate was almost completely soluble in Bray-1 reagent yet it was insoluble in Morgan reagent. P sorbed by calcium carbonate was insoluble in acid Bray-1 reagent but soluble in the alkaline Colwell soil test. In the presence of soil, the amounts of P extracted from the compounds were commonly reduced by sorption of dissolved P by the soil. For example, most dissolved P was sorbed by the soil from an ammonium sulphate solution whereas relatively little P was sorbed by soil from the Bray-1 reagent. The strong influence of the type of P compound on the solubility of P in soil test reagents might be partly responsible for the need for different calibration curves of P soil test versus plant yield for soils that have received different types of P fertilizer.
Similar content being viewed by others
References
Bray RH and Kurtz LT (1945) Determination of total organic and available forms of phosphorus in soils. Soil Sci 59: 39–45
Charleston AG, Condron LM and Brown IWM (1989) The nature of the residual apatites remaining after partial acidulation of phosphate rocks with phosphoric and sulphuric acids. Fert Res 18: 257–273
Colwell JD (1963) The estimation of phosphorus fertilizer requirements of wheat in southern New South Wales by soil analysis. Aust J Expt Agri Anim Husb 3: 190–197
Dean LA and Rubins EJ (1947) Anion exchange in soils. 1. Exchangeable phosphorus and the anion exchange capacity. Soil Sci 63: 377–387
Dickman SR and Bray RH (1940) Colorimetric determination of phosphate. Ind Eng Chem Anal Ed 12: 665–668
Gilkes RJ and Palmer B (1979) Calcined Christmas Island C-grade rock phosphate fertilizers: mineralogical properties, reversion and assessment by chemical extraction. Aust J Soil Res 17: 467–481
Gilkes RJ and Mangano P (1983) Poorly soluble, ironaluminium phosphates in ammonium phosphate fertilizers: their nature and availability to plants. Aust J Soil Res 21: 193–194
Gilkes RJ and Lim-Nunez R (1980) Poorly soluble phosphates in Australian superphosphate: their nature and availability to plants. Aust J Soil Res 31: 85–95
Grigg JL (1965) Inorganic phosphorus fractions in South Island soils and their solubility in commonly used extracting solutions. NZ J Agric Res 8: 313–326
Hingston FJ (1970) Specific adsorption of anions on goethite and gibbsite. PhD Thesis Univ West Aust Nedlands Western Australia
Hingston FJ, Posner AM and Quirk JP (1972) Anion adsorption by goethite and gibbsite. 1. The role of the proton in determining adsorption envelopes. J Soil Sci 23: 177–192
Hughes JC and Gilkes RJ (1986) The effect of rock phosphate properties on the extent of fertilizer dissolution in soils. Aust J Soil Res 22: 475–481
Joret G and Hebert J (1955) Contribution a la determination du besoin des sols en acide phosphorique. Annales Agronomiques 6: 233–299
Kamprath EJ and Watson ME (1980) Conventional soil and tissue tests for assessing the phosphorus status of soils. pp 433–469 In: Khasawneh FE, Sample EC and Kamprath EJ (eds) The role of Phosphorus in Agriculture. Am Soc Agron. Madison, USA
Kumar V, Gilkes RJ and Bolland MDA (1991) Residual phosphate fertilizer compounds in soils. I. Their estimation using selective extractants. Fert Res 30: 19–29
Kumar V, Gilkes RJ and Bolland MDA (1991) Residual phosphate fertilizer compounds in soils. III. Their effect on the relationship between yield and soil test values. Fert Res (submitted)
Morgan MF (1941) Chemical soil diagnosis by the universal testing system. Conn Agric Exp Stn Bull 450
Olsen SR, Cole CV, Watanabe FS and Dean LA (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA circ no 939. Washington DC
Riehm H (1955) Methodenbuch Band I Die Untersuchung von Boden Thun R, Herrmann R and Knickmann E (eds) Neumann Verlag, Berlin
Sibbesen E (1983) Phosphate soil tests and their suitability to assess the phosphate status of soil. J Sci Food Agri 34: 1368–1374
Smillie GW and Syers JK (1972) Calcium fluoride formation during extraction of calcareous soils with fluoride: II Implication to Bray P-1 test. Soil Sci Soc Amer Proc 36: 25–30
Stahlberg S (1980) A new extraction method for estimation of plant available P, K and Mg. A trial application in Swedish cultivated soils. Acta Agricultura Scandinavica 30: 93–107
Thomas GW and Peaslee DE (1973) Testing soil for phosphorus. In: Walsh LM and Beaton JD (eds) Soil Testing and Plant Analysis. Soil Sci Soc Am Madison, USA.
Truog E (1930) The determination of the readily available phosphorus of soil. J Am Soc Agron 22: 874–882
Tyner EH and Davide JG (1962) Some criteria for evaluating phosphorus tests for lowland rice soils. pp 625–634 In Neale GJ (ed) Trans Comm IV and V Int Soc of Soil Sci Palmerston North New Zealand. Soil Bureau Lower Hutt New Zealand
Watanabe FS and Olsen SR (1965) Test of an ascorbic acid method for determining phosphorus in water and NaHCO3 extracts from soil. Soil Sci Soc Amer Proc 9: 677–678
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kumar, V., Gilkes, R.J. & Bolland, M.D.A. Residual phosphate fertilizer compounds in soils. Fertilizer Research 30, 31–38 (1991). https://doi.org/10.1007/BF01048824
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01048824