Abstract
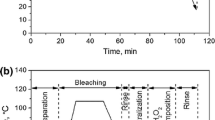
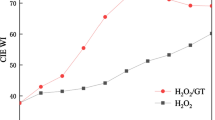
A closed system bleaching apparatus was designed to determine the kinetics and effects of various factors on alkaline hydrogen peroxide bleaching of textile cellulose fabrics. It was confirmed that perhydroxyl anion is the primary bleaching moiety in alkaline hydrogen peroxide systems. The use of the apparatus in the measurement of fabric color, waste oxygen, and the subsequent calculation of hydroxyl ion, and molecular hydrogen peroxide confirmed that pH and titration of 'free' hydrogen peroxide in alkaline bleaching systems are not good indicators of bleaching mechanism. The role of the cellulose itself in the chemical bleaching system was determined. The rate of bleaching on cotton fabric was shown to be a first order reaction in concentration of perhydroxyl anion at 60 and 90 °C. An activation energy of 17 kcal/mole was estimated. Decomposition of H2O2 into waste oxygen was found to be second order kinetics.
Similar content being viewed by others
References
Taher, A. M. M. and Cates, D. M. (1975) Bleaching cellulose: Part I. A free radical mechanism. Textile Chemist & Colorist 7, 220-224.
Steinmiller, W. G. and Cates, D. M. (1976) Bleaching cellulose: Part II. Effect of impurities. Textile Chemist & Colorist 8, 30-34.
Dannacher, J. and Schlenker, W. (1996) The mechanism of hydrogen peroxide bleaching. Textile Chemist & Colorist 28(11), 24-28.
Duke, F. R. and Haas, T. W. (1961) The homogeneous base-catalyzed decomposition of hydrogen peroxide. J. Phy. Chem. 65, 304-306.
Spiro, M. and Griffith, W. P. (1997) The mechanism of hydrogen peroxide bleaching. Textile Chemist & Colorist 29(11), 12-13.
Thompson, K. M., Griffith, W. P. and Spiro, M. (1993) (1994) (1999) Mechanism of bleaching by peroxides Part 1, 2, and 3. J. Chem. Soc., Faraday Trans. 89, 1203-1209; J. Chem. Soc., Faraday Trans. 90, 1105-1114; J. Chem. Soc., Faraday Trans. 89, 4035-4043.
Simon, S. A. and Drelich, Arthur (1946) Oxygen balance in peroxide bleaching. Textile Res. J. 16, 609-615.
Milne, N. V. (1998) Oxygen bleaching systems in domestic laundry. J. Surfactants and Detergents 1, 253-261.
Isbell, H. S., Frush, H. L., Naves, P. and Soontracharoen, P. (1981) Degradation of 2-deoxyaldoses. Carbohydrate Res. 90, 111-122.
Galbacs, Z. M. and Gangi, L. V. (1983) Alkali-Induced decomposition of hydrogen peroxide. J. Chem. Soc., Dalton Trans. 2353-2357.
Koubek, E., Haggett, M. L., Bathaglia, C. S., Ibne-Rasa, K. M., Pyun, H. Y. and Edwards, O. J. (1963) Kinetics and mechanism of the spontaneous decompositions of some peroxocids, hydrogen peroxide and t-butyl hydroperoxide. J. Chem. Soc. 85, 2263-2268.
Goodman, J. F., Robson, P. and Wilson, E. R. (1962) Decomposition of aromatic peroxyacids in aqueous alkali. Trans. Faraday Soc. 58, 1846-1851.
Evans, M. G. and Uri, N. (1949) The dissociation constant of hydrogen peroxide. Trans. Faraday Soc. 45, 224-230.
Evans, D. F. and Upton, M. W. (1985) The spontaneous and catalyzed decomposition of hydrogen peroxide. J. Chem. Soc., Dalton Trans. 2526-2529.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brooks, R.E., Moore, S.B. Alkaline hydrogen peroxide bleaching of cellulose. Cellulose 7, 263–286 (2000). https://doi.org/10.1023/A:1009273701191
Issue Date:
DOI: https://doi.org/10.1023/A:1009273701191