Summary
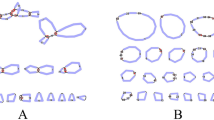
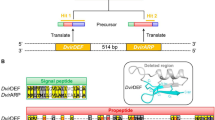
Single-fly polymerase chain reaction amplification and direct DNA sequencing revealed high levels of length polymorphism in the threonine-glycine encoding repeat region of theperiod (per) gene in natural populations ofDrosophila melanogaster. DNA comparison of two alleles of identical lengths gave a high number of synonymous substitutions suggesting an ancient time of separation. However detailed examination of the sequences of different Thr-Gly length variants indicated that this divergence could be understood in terms of four deletion/insertion events. InDrosophila pseudoobscura a length polymorphism is observed in a five-amino acid degenerate repeat, which corresponds tomelanogaster's Thr-Gly domain. In spite of the differences betweenD. melanogaster andD. pseudoobscura in the amino acid sequence of the repeats, the predicted secondary structures suggest evolutionary and mechanistic constraints on theper protein of these two species.
Similar content being viewed by others
References
Anderson PR, Oakeshott JG (1984) Parallel geographic patterns of allozyme variation in two siblingDrosophila species. Nature 308:729–731
Ayala FJ, Dobzhansky T (1974) A new subspecies ofDrosophila pseudoobscura (Diptera: Drosophilidae). Pan-Pac Entomol 50: 211–219
Bachmann B, Luke W, Hunsmann G (1990) Improvement of PCR amplified DNA sequencing with aid of detergents. Nucleic Acids Res 18:1309
Burgess AW, Ponnuswamy PK, Scheraga HA (1974) Analysis of conformation of amino acid residues and prediction of backbone topography in proteins. Isr J Chem 12:239–286
Chou PY, Fasman GD (1974) Predictions of protein conformation. Biochemistry 13:222–245
Citri Y, Colot HV, Jacquier AC, Yu Q, Hall JC, Baltimore D, Rosbash M (1987) A lamily of unusually spliced biologically active transcripts encoded by aDrosophila clock gene. Nature 326:42–47
Colot HV, Hall JC, Rosbash M (1988) Interspecific comparison of theperiod gene ofDrosophila reveals large blocks of nonconserved coding DNA. EMBO J 7:3929–3937
David JR, Alonso-Moraga A, Borai F, Capy P, Mercot H, McEvey SF, Munoz-Serrano A, Tsakas S (1989) Latitudinal variation ofAdh gene frequencies inDrosophila melanogaster: a Mediterranean instability. Heredity 62:11–16
Devereux J, Haeberli P, Smithies O (1984) A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res 12:387–396
Dover GA (1987) DNA turnover and the molecular clock. J Mol Evol 26:47–58
Dover GA (1989) Slips, strings and species. Trends Genet 5: 100–102
Dufton MJ, Hider RC (1977) Snake toxin secondary structure predictions. Structure activity relationships. J Mol Biol 115: 177–193
Ewer J, Hamblen-Coyle M, Rosbash M, Hall JC (1990) Requirement forperiod gene expression in the adult and not during development for locomotor activity rhythms of imaginalDrosophila melanogasten. J Neurogenet 7:31–73
Garnier J, Osguthorpe DJ, Robson B (1978) Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol 120: 97–120
Goodbourn SEY, Higgs DR, Clegg JB, Weatherall DJ (1983) Molecular basis of length polymorphism in the human ζ-globin gene complex. Proc Natl Acad Sci USA 80:5022–5026
Hall JC, Kyriacou CP (1990) Genetics of biological rhythms inDrosophila. Adv Insect Physiol 22:221–298
Jackson FR, Bargiello TA, Yun S-H, Young MW (1986) Product of theper locus ofDrosophila shares homology with proteoglycans. Nature 320:185–188
Jarman AP, Wells RA (1989) Hypervariable minisatellites: recombinators or innocent bystanders? Trends Genet 5:367–371
Jeffreys AJ, Royle NJ, Wilson V, Wong Z (1988a) Spontaneous mutation rates to new length alleles at tandem-repetitive hypervariable loci in human DNA. Nature 332:278–281
Jeffreys AJ, Wilson V, Neumann R, Keyte J (1988b) Amplification of human minisatellites by polymerase chain reaction: towards DNA fingerprinting of single cells. Nucleic Acids Res 16:10953–10971
Jeffreys AJ, Neumann R, Wilson V (1990) Repeat unit sequence variation in minisatellites: a novel source of DNA polymorphism for studying variation and mutation by single molecule analysis. Cell 60:473–485
Jowett T (1986) Preparation of nucleic acids. In: Roberts DB (ed)Drosophila. A practical approach. IRL Press, Oxford, pp 275–286
Kabat EA, Wu TT (1973) The influence of nearest-neighbour amino acids on the conformation of the middle amino acid in proteins: comparisons of predicted and experimental determination of β-sheets in concanavalin A. Proc Natl Acad Sci USA 70:1473–1477
Kyriacou CP (1990) The molecular ethology of theperiod gene inDrosophila. Behav Genet 20:191–211
Levinson G, Gutman GA (1987) Slipped-strand mispairing: a major mechanism for DNA sequence evolution. Mol Biol Evol 4:203–221
Lim VI (1974) Structural principles of the globular organization of protein chains. A stereochemical theory of globular protein secondary structure. J Mol Biol 88:857–872
Lindsley DL, Grell EH (1972) Genetic variations ofD. melanogaster. Carnegie Inst Washington Publ 627:1–471
Lyons KM, Stein JH, Smithies O (1988) Length polymorphism in human proline-rich protein genes generated by intragenic unequal crossing-over. Genetics 120:267–278
McLachlan AD (1977) Quantum chemistry and protein folding: the art of the possible. Int J Quantum Chem [Suppl 1] 12: 371–385
Muskavitch MAT, Hogness DS (1982) An expandable gene that encodes aDrosophila glue protein is not expressed in variants lacking remote upstream sequences. Cell 29:1041–1051
Nagano K (1973) Logical analysis of the mechanism of protein folding. I. Predictions of helices, loops and β structures from primary structure. J Mol Biol 75:401–420
Orr HA (1989) The genetics of sterility in hybrids between two subspecies ofDrosophila. Evolution 43:180–189
Pittendrigh CS (1954) On the temperature independence in the clock system controlling emergence time inDrosophila. Proc Natl Acad Sci USA 40:1018–1029
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467
Smith GP (1976) Evolution of repeated DNA sequences by unequal crossover. Science 191:528–535
Swallow DM, Gendler S, Taylor-Papadimitriou J, Bramwell ME (1987) The human tumor-associated epithelial mucins are coded by an expressed hypervariable gene locus PUM. Nature 328:82–84
Tautz D, Trick M, Dover GA (1986) Cryptic simplicity in DNA is a major source of genetic variation. Nature 322:652–656
Teumer J, Green H (1989) Divergent evolution of part of the involucrin gene in the hominoids: unique intragenic duplications in the gorilla and human. Proc Natl Acad Sci USA 86:1283–1286
Thackeray JR, Kyriacou CP (1990) Molecular evolution in theDrosophila yakuba period locus. J Mol Evol 31:389–401
Treier M, Pfeifle C, Tautz D (1989) Comparison of the gap segmentation genehunchback betweenDrosophila melanogaster andDrosophila virilis reveals novel models of evolutionary change. EMBO J 8:1517–1525
Wheeler DA, Kyriacou CP, Greenacre M, Yu Q, Rutila J, Rosbash M, Hall JC (1991) Molecular transfer of a species-specific courtship behaviour fromDrosophila simulans toDrosophila melanogaster. Science (in press)
Yu Q, Colot HV, Kyriacou CP, Hall JC, Rosbash M (1987) Behaviour modification by in vitro mutagenesis of a variable region within theperiod gene ofDrosophila. Nature 326:765–769
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Costa, R., Peixoto, A.A., Thackeray, J.R. et al. Length polymorphism in the threonine-glycine-encoding repeat region of theperiod gene inDrosophila . J Mol Evol 32, 238–246 (1991). https://doi.org/10.1007/BF02342746
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02342746