Abstract
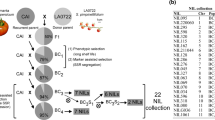
The usefulness of marker-assisted selection (MAS) to develop salt-tolerant breeding lines from a F2 derived from L. esculentum x L. pimpinellifolium has been studied. Interval mapping methodology of quantitative trait locus (QTL) analysis was used to locate more precisely previously detected salt tolerance QTLs. A new QTL for total fruit weight under salinity (TW) near TG24 was detected. Most of the detected QTLs [3 for TW, 5 for fruit number, (FN) and 4 for fruit weight (FW)] had low R 2 values, except the FW QTL in the TG180-TG48 interval, which explains 36.6% of the total variance. Dominant and overdominant effects were detected at the QTLs for TW, whereas gene effects at the QTLs for FJV and FW ranged from additive to partial dominance. Phenotypic selection of F2 familes and marker-assisted selection of F3 families were carried out. Yield under salinity decreased in the F2 generation. F3 means were similar to those of the F1 as a consequence of phentoypic selection. The most important selection response for every trait was obtained from the F3 to F4 where MAS was applied. While F3 variation was mainly due to the within-family component, in the F4 the FN and FW between-family component was larger than the within-family one, indicating an efficient compartmentalization and fixation of QTLs into the F4 families. Comparison of the yield of these families under control versus saline conditions showed that fruit weight is a key trait to success in tomato salt-tolerance improvement using wild Lycopersicon germplasm. The QTLs we have detected under salinity seem to be also working under control conditions, although the interaction family x treatment was significant for TW, thereby explaining the fact that the selected families responded differently to salinity.
Similar content being viewed by others
References
Asharf M, McNeilly (1990) Improvement of salt tolerance in maize by selection and breeding. Plant Breed 104:101–107
Asíns MJ, Carboneil EA (1988) Detection of linkage between restriction fragment length polymorphism markers and quantitative traits. Theor Appl Genet 76:623–626
Asíns MJ, Bretó MP, Cambra M, Carbonell EA (1993) Salt tolerance in Lycopersicon species. II. Genetic effects and search for associated traits. Theor Appl Genet 86:769–774
Asins MJ, Mestre P, García JE, Dicenta F, Carbonell EA (1994) Genotype x environment interaction in QTL analysis of an intervarietal almond cross by means of genetics markers. Theor Appl Genet 89:358–364
Beckman JS, Soller M (1983) Restriction fragment length polymorphism in genetic improvement methodologies, mapping and cost. Theor Appl Genet 67:35–43
Bosemark NO (1994) Plant breeding in a changing agriculture. In: Martín Sánchez JA, Fibla J, Aldea M (eds) Proc 29th Jornadas Genét Luso-Españolas. Ediciones de la Universidad de Lleida, Lleida, Spain. pp vi3-vi5
Botstein D, White RL, Skolnich M, Davis RW (1980) Construction of a genetic map in man using restriction length polymorphism. Am J Hum Genet 32:314–331
Bretó MP, Asíns MJ, Carbonell EA (1994) Salt tolerance in Lycopersion species. III. Detection of QTLs by means of molecular markers. Theor Appl Genet 88:395–401
Carbonell EA, Asins MJ (1996) Statistical models for the detection of genes controlling quantitative trait loci expression. In:Jain SM, Sopory SK, Veilleux RE (eds) In vitro haploid production in higher plants, vol. 2. Kluwer Academis Publ, The Netherlands. pp 255–280
Carbonell EA, Gerig TM (1991) A program to detect linkage between genetic markers and nonadditive QTLs. J Hered 82:435
Carbonell EA, Gerig TM, Balansard E, Asíns MJ (1992) Interval mapping in the analysis of non-aditive quantitative trait loci. Biometrics 48:305–315
Carbonell EA, Asins MJ, Baselga M, Balansard E, Gerig TM (1993) Power studies in the estimation of genetic parameters and the localization of quantitative trait loci for backcross and doubled haploid populations. Theor Appl Genet 86:411–416
Cuartero J, Yeo AR, Flowers TJ (1992) Selection of donors for salt-tolerance in tomato using physiological traits. New Phytol 121:63–69
Darvasi A, Soller M (1994) Optimum spacing of genetic markers for determining linkage between marker loci and quantitative trait loci. Theor Appl Genet 89:351–357
Edwards MD, Page NJ (1994) Evaluation of marker-assisted selection through computer simulation. Theor Appl Genet 88:376–382
Edwards MD, Stuber CW, Wendel JF (1987) Molecular-marker-facilitated investigations of quantitative trait loci in maize I. Numbers, genomic distribution and types of gene action. Genetics 166:113–125
Falconer SD (1989) Introduction to quantitative genetics. Longman and John Wiley & Sons, New York
Hyne V, Kearsey MJ, Pike DJ, Snape JW (1995) QTL analysis: unreliability and bias in estimation procedures. Mol Breed 1:273–282
Jensen RC, van Ooijen JW, Stam P, Lister C, Dean C (1995) Genotype by environment interaction in genetic mapping of multiple quantitative trait loci. Theor Appl Genet 91:33–37
Jones RA (1986) The development of salt-tolerant tomatoes: breeding strategies. Acta Hortic 190:101–114
Kosambi DD (1944) The estimation of map distances from recombination values. Ann Eugen 12:172–175
Lande R (1992) Marker-assisted selection in relation to traditional methods of plant breeding. In: Stalker HT, Murph JP (eds) Plant breeding in the 1990s. CAB Int, Wallingford, UK, pp 437–541
Lande R, Thompson R (1990) Efficiency of marker-assisted selection in the improvement of quantitative traits. Genetics 124:743–756
Lander ES, Botstein D (1989) Mapping Mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics 121:185–189
Lander ES, Green P, Abrahanson J, Ballow A, Daley M, Lincoln S, Newburg L (1987) MAPMAKER:an interactive computer program package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1:174–181
Lark KG, Orf J, Mansur LM (1994) Epistatic expression of quantitative trait loci (QTL) in soybean (Glycine max L. Mer.) determined by QTL association with RFLP alleles. Theor Appl Genet 88:486–489
Maas EW, Hoffman GJ (1977) Crop salt tolerance-current assesment. J Irrig Drai Div Am Soc Civil Eng 103:115–134
Mather K, Jinks JL (1971) Biometrical genetics. Chapman and Hall, London
Nyquist WE (1991) Estimation of heritability and prediction of selection response in plant populations. CRC Plant Sci 10:235–322
Paterson AH, Lander ES, Hewitt JD, Peterson S, Lincoln SE, Tanksley SD (1988) Resolution of quantitative traits into Mendelian factors by using a complete linkage map of restiction fragment polymorphisms. Nature 335:721–726
Paterson AH, Damon S, Hewitt JD Zamir D, Rabinowitch HD, Lincoln SE, Lander ES, Tanksley SD (1991) Mendelian factors underlying quantitative traits in tomato: comparisions across species, generations and environments. Genetics 127:181–197
Rebaï A, Goffinet B, Mangin B (1994) Aproximative thresholds of interval mapping test for QTL detection. Genetics 138:235–240
Saranga Y, Zamir D, Marani A, Rudich (1991) Breeding tomatoes for salt tolerance:field evaluation of Lycopersicon germplasm for yield and dry-matter production. J Am Soc Hortic Sci 116:1067–1071
Stromberg LD, Dudley JW, Rufener GK (1994) Comparing conventional early generation selection with molecular marker assisted selection in maize. Crop Sci 34:1221–1225
Stuber CW, Lincoln SE, Wolff DW, Helentjaris T, Lander ES (1992) Identification of genetic factors contributing to heterosis in a hybrid from two elite maize inbred lines using molecular makers. Genetics 132:823–839
Tanksley SD (1993) Mapping poligenes. Annu Rev Genet 27:205–233
Tanksley SD Ganal MW, Prince JP, de Vicente MC, Bonierbale MW, Broun P, Fulton TN, Giovannoni JJ, Grandillo S, Martin GB, Messeguer R, Miller JC, Miller L, Paterson AH, Pineda O Röder MS, RA Winig, Wu W, Young ND (1992) High density molecular linkage maps of the tomato and potato genomes. Genetics 132:1141–1160
de Vicente MC, Tanksley SD (1993) QTL analysis of transgressive segregation in an interspecific tomato cross. Genetics 134:585–596
Uzunova M, Ecke W, Weissleder K, Röbbelen (1995) Mapping the genome of rapeseed (Brassica napus L.) I. Construction of a RFLP linkage map and localization of QTLs for seed glucosinolate content. Theor Appl Genet 90:194–204
Welsh J, McClelland M (1990) Fingerprinting genomes using the PCR arbitrary primers. Nucleic Acids Res 18:7213–7218
Williams JGK, Kubelik AR, Livak KJ, Rafalski JA, Tingey SV (1990) DNA polymorphism amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res 18:6531–6535
Zhang W, Smith (1992) Computer simulation of marker-assisted selection utilizing linkage disequilibrium. Theor Appl Genet 83:813–820
Author information
Authors and Affiliations
Additional information
Communicated by P. M. A. Tigerstedt
Rights and permissions
About this article
Cite this article
Monforte, A.J., Asíns, M.J. & Carbonell, E.A. Salt tolerance in Lycopersicon species. IV. Efficiency of marker-assisted selection for salt tolerance improvement. Theoret. Appl. Genetics 93, 765–772 (1996). https://doi.org/10.1007/BF00224074
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00224074