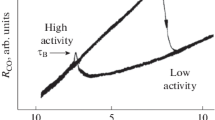
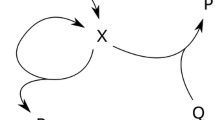
Abstract
The kinetics of a catalytic reaction is frequently formulated in terms of simple concepts of the Langmuir type. Apart from limitations arising from the non-uniformity of the catalyst's surface and from the coverage dependence of the rate “constants”, several other complications may come into play. These may arise on the “quantum level” where energy flow between the various degrees of freedom may cause failure of simple transition state theory, as well as on the “continuum level” where formulation of rate equations in terms of coupled non-linear differential equations may give rise to a rich scenario of spatio-temporal self-organization, including kinetic oscillations, chaos, and formation of concentration patterns. Several of these phenomena are illustrated by selected examples.
Similar content being viewed by others
References
J. A. Dumesic, contribution at this meeting;
P. Stoltze and J.K. Nørskov, Phys. Rev. Lett. 55 (1985) 2502; Surf. Sci. 197 (1988) L230; J. Catal. 110 (1988) 1;
M. Bowker, I. Parker and K.C. Waugh, Appl. Catal. 14 (1985) 101; Surf. Sci. 97(1988)L223;
J.A. Dumesic and A. A. Treviño, J. Catal. 116 (1989) 119;
J.W. Geus and K.C. Waugh, in:Catalytic Ammonia Synthesis, ed. J.R. Jennings (Plenum Press, New York, 1991) p. 179.
G. Ertl, S.B. Lee and M. Weiss, Surf. Sci. 114 (1982) 515.
C.T. Rettner and H. Stein, Phys. Rev. Lett. 59 (1987) 2768.
M.C. Tsai, U. Seip, I. Bassignana, J. Küppers and G. Ertl, Surf. Sci. 155 (1985) 387.
H. Shi, K. Jacobi and G. Ertl, J. Chem. Phys., submitted.
T. Matsushima, Surf. Sci. 197 (1988) L287.
G. Doyen, Vacuum 32 (1982) 91.
H. Brune, J. Wintterlin, R.J. Behm and G. Ertl, Phys. Rev. Lett. 68 (1992) 624.
A.F. Carley and M.W. Roberts, J. Chem. Soc. Chem. Comm. (1987) 355.
T. Matsushima, Surf. Sci. 127 (1983) 403.
W.D. Mieher and W. Ho, J. Chem. Phys. 91 (1989) 2755.
T. Gritsch, D. Coulman, R.J. Behm and G. Ertl, Phys. Rev. Lett. 63 (1989) 1086.
K. Krischer, M. Eiswirth and G. Ertl, J. Chem. Phys. 96 (1992) 9161.
M. Eiswirth, T.M. Kruel, G. Ertl and F.W. Schneider, Chem. Phys. Lett. 193 (1992) 305.
W. Engel, M. Kordesch, H.H. Rotermund, S. Kubala and A. von Oertzen, Ultramicroscopy 36 (1991) 148.
G. Ertl, Science 254 (1991) 1750.
S. Nettesheim, A. von Oertzen, H.H. Rotermund and G. Ertl, J. Chem. Phys. 98 (1993) 9977.
J. Lauterbach, G. Haas, H.H. Rotermund and G. Ertl, Surf. Sci., in press.
V. Gorodetskii, W. Drachsel and J.H. Block, Catal. Lett. 19 (1993) 223;
V. Gorodetskii, J.H. Block, W. Drachsel and M. Ehsasi, Appl. Surf. Sci. 67 (1993) 198.
M. Bär, Ch. Zülicke, M. Eiswirth and G. Ertl, J. Chem. Phys. 96 (1992) 8595.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ertl, G. Dynamics and self-organization of catalytic systems. Top Catal 1, 305–314 (1994). https://doi.org/10.1007/BF01492284
Issue Date:
DOI: https://doi.org/10.1007/BF01492284