Abstract
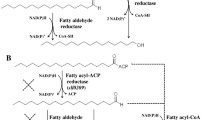
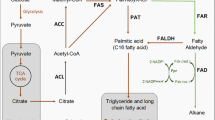
The production of alkanes in a marine cyanobacterium possessing the α-olefin biosynthesis pathway was achieved by introducing an exogenous alkane biosynthesis pathway. Cyanobacterial hydrocarbons are synthesized via two separate pathways: the acyl-acyl carrier protein (ACP) reductase/aldehyde-deformylating oxygenase (AAR/ADO) pathway for the alkane biosynthesis and the α-olefin synthase (OLS) pathway for the α-olefin biosynthesis. Coexistence of these pathways has not yet been reported. In this study, the marine cyanobacterium Synechococcus sp. NKBG15041c was shown to produce α-olefins similar to those of Synechococcus sp. PCC7002 via the α-olefin biosynthesis pathway. The production of heptadecane in Synechococcus sp. NKBG15041c was achieved by expressing the AAR/ADO pathway genes from Synechococcus elongatus PCC 7942. The production yields of heptadecane in Synechococcus sp. NKBG15041c varied with the expression level of the aar and ado genes. The maximal yield of heptadecane was 4.2 ± 1.2 μg/g of dried cell weight in the transformant carrying a homologous promoter. Our results also suggested that the effective activation of ADO may be more important for the enhancement of alkane production by cyanobacteria.




Similar content being viewed by others
References
Bagdasarian M, Lurz R, Ruckert B, Franklin FC, Bagdasarian MM, Frey J, Timmis KN (1981) Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene 16(1–3):237–247
Coates RC, Podell S, Korobeynikov A, Lapidus A, Pevzner P, Sherman DH, Allen EE, Gerwick L, Gerwick WH (2014) Characterization of cyanobacterial hydrocarbon composition and distribution of biosynthetic pathways. PLoS ONE 9(1):e85140
Hiroe A, Tsuge K, Nomura CT, Itaya M, Tsuge T (2012) Rearrangement of gene order in the phaCAB operon leads to effective production of ultrahigh-molecular-weight poly [(R)-3-hydroxybutyrate] in genetically engineered Escherichia coli. Appl Environ Microbiol 78(9):3177–3184
Hu P, Borglin S, Kamennaya NA, Chen L, Park H, Mahoney L, Kijac A, Shan G, Chavarria KL, Zhang CM, Quinn NWT, Wemmer D, Holman HY, Jansson C (2013) Metabolic phenotyping of the cyanobacterium Synechocystis 6803 engineered for production of alkanes and free fatty acids. Appl Energ 102:850–859. doi:10.1016/j.apenergy.2012.08.047
Huang HH, Camsund D, Lindblad P, Heidorn T (2010) Design and characterization of molecular tools for a synthetic biology approach towards developing cyanobacterial biotechnology. Nucleic Acids Res 38(8):2577–2593
Kaiser BK, Carleton M, Hickman JW, Miller C, Lawson D, Budde M, Warrener P, Paredes A, Mullapudi S, Navarro P (2013) Fatty aldehydes in cyanobacteria are a metabolically flexible precursor for a diversity of biofuel products. PLoS ONE 8(3):e58307
Kloft N, Rasch G, Forchhammer K (2005) Protein phosphatase PphA from Synechocystis sp. PCC 6803: the physiological framework of PII-P dephosphorylation. Microbiology 151(4):1275–1283
Knothe G (2010) Biodiesel and renewable diesel: a comparison. Prog Energy Combust Sci 36(3):364–373
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23(21):2947–2948. doi:10.1093/bioinformatics/btm404
Lennen RM, Pfleger BF (2013) Microbial production of fatty acid-derived fuels and chemicals. Curr Opin Biotechnol 24(6):1044–1053
Li N, Chang WC, Warui DM, Booker SJ, Krebs C, Bollinger JM (2012) Evidence for only oxygenative cleavage of aldehydes to alk(a/e)nes and formate by cyanobacterial aldehyde decarbonylases. Biochemistry 51(40):7908–7916. doi:10.1021/Bi300912n
Liu A, Zhu T, Lu X, Song L (2013) Hydrocarbon profiles and phylogenetic analyses of diversified cyanobacterial species. Appl Energ 111:383–393
Matsunaga T, Takeyama H (1995) Genetic-engineering in marine cyanobacteria. J Appl Phycol 7(1):77–84. doi:10.1007/Bf00003555
Mendez-Perez D, Begemann MB, Pfleger BF (2011) Modular synthase-encoding gene involved in α-olefin biosynthesis in Synechococcus sp. strain PCC 7002. Appl Environ Microbiol 77(12):4264–4267
Ruffing AM (2011) Engineered cyanobacteria: teaching an old bug new tricks. Bioeng Bugs 2(3):136–149
Sakamoto T, Higashi S, Wada H, Murata N, Bryant DA (1997) Low‐temperature‐induced desaturation of fatty acids and expression of desaturase genes in the cyanobacterium Synechococcus sp. PCC 7002. FEMS Microbiol Lett 152(2):313–320
Schirmer A, Rude MA, Li X, Popova E, Del Cardayre SB (2010) Microbial biosynthesis of alkanes. Science 329(5991):559–562
Sode K, Tatara M, Takeyama H, Burgess JG, Matsunaga T (1992) Conjugative gene transfer in marine cyanobacteria: Synechococcus sp., Synechocystis sp. and Pseudanabaena sp. Appl Microbiol Biotechnol 37(3):369–373
Sode K, Tatara M, Hatano N, Matsunaga T (1994) Foreign gene expression in marine cyanobacteria under pseudo-continuous culture. J Biotechnol 33(3):243–248
Sode K, Yamamoto Y, Hatano N (1998) Construction of a marine cyanobacterial strain with increased heavy metal ion tolerance by introducing exogenic metallothionein gene. J Mar Biotechnol 6(3):174–177
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28(10):2731–2739. doi:10.1093/molbev/msr121
Vidal R, López-Maury L, Guerrero MG, Florencio FJ (2009) Characterization of an alcohol dehydrogenase from the cyanobacterium Synechocystis sp. strain PCC 6803 that responds to environmental stress conditions via the Hik34-Rre1 two-component system. J Bacteriol 191(13):4383–4391
Wang W, Liu X, Lu X (2013) Engineering cyanobacteria to improve photosynthetic production of alka(e)nes. Biotechnol Biofuels 6(1):69
Wilmotte A, Herdman M (2001) Phylogenetic relationships among the cyanobacteria based on 16S rRNA sequences. Bergey’s Manual of Systematic Bacteriology Volume One: The Archaea and the Deeply Branching and Phototrophic Bacteria
Winters K, Parker PL, Van Baalen C (1969) Hydrocarbons of blue-green algae: geochemical significance. Science 163(3866):467–468. doi:10.1126/science.163.3866.467
Yoshino T, Honda T, Tanaka M, Tanaka T (2013) Draft genome sequence of marine cyanobacterium Synechococcus sp. Strain NKBG15041c. Genome announcements 1(6) doi:10.1128/genomeA.00954-13
Yu R, Yamada A, Watanabe K, Yazawa K, Takeyama H, Matsunaga T, Kurane R (2000) Production of eicosapentaenoic acid by a recombinant marine cyanobacterium, Synechococcus sp. Lipids 35(10):1061–1064
Zhang J, Lu X, Li J-J (2013) Conversion of fatty aldehydes into alk(a/e)nes by in vitro reconstituted cyanobacterial aldehyde-deformylating oxygenase with the cognate electron transfer system. Biotechnol Biofuels 6(1):86
Acknowledgments
This study was supported by JST, CREST.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 857 kb)
Rights and permissions
About this article
Cite this article
Yoshino, T., Liang, Y., Arai, D. et al. Alkane production by the marine cyanobacterium Synechococcus sp. NKBG15041c possessing the α-olefin biosynthesis pathway. Appl Microbiol Biotechnol 99, 1521–1529 (2015). https://doi.org/10.1007/s00253-014-6286-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6286-2